Which Diagram Is A Bomb Calorimeter
A parrtm model 1108 oxygen combustion vessel as used in this experi ment. Bomb calorimeter a device for measuring the energy content of material.
 Calorimeter Instrument Britannica Com
Calorimeter Instrument Britannica Com
A bomb calorimeter measures heat for liquid products only.
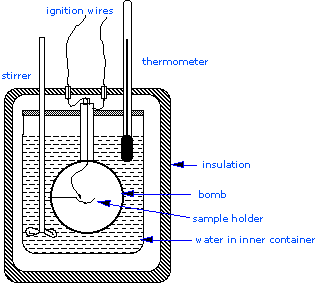
Which diagram is a bomb calorimeter. In a bomb calorimeter the reaction takes place quickly. The calorimeter has a mass of 2000 kg and a specific heat of 295 jg c. The parr bomb is a bomb calorimeter a type of constant volume calorimeter as opposed to typical styrofoam cup calorimeters which are constant pressure calorimeters at least in theory.
As the fuel is burning it will heat up the surrounding air which expands and escapes through a tube that leads the air out of the calorimeter. The temperature of the water bath can be monitored via thermometer. Overall however the reactions are the same and the amount of energy released is comparable.
Alright so lets talk about a bomb calorimeter. The quantity of heat that is required to raise the samples temperature by 1c or kelvin a 120 g sample of propane c3h8 is burned in a bomb calorimeter. The temperature of the calorimeter rises from 200c to 300c.
A bomb calorimeter is used for measuring energy released in a combustion reaction. The sample is burned in an oxygen rich atmosphere inside a sealed chamber that is surrounded by a jacket containing a known volume of water. A bomb calorimeter has a separate chamber to hold substances and can even measure heat gain or loss for reactions that do not occur in water.
A 120 g sample of propane c3h8 is burned in a bomb calorimeter. The temperature of the calorimeter rises from 200c to 300c. How does a bomb calorimeter work.
Electrical energy is used to ignite the fuel. Inside the calorimeter is a vessel in which the reaction occurs surrounded by a water bath. When the air is escaping through the copper tube it will also heat up the water outside the tube.
This reaction takes place in a water bath so that the water absorbs the energy released and we can measure how much its temperature rises accordingly. A bomb calorimeter can measure heat gain or loss in gaseous reactions but is not useful for reactions that occur at high pressures and temperatures. The calorimeter has a mass of 2000 kg and a specific heat of 295 jg c.
30th sep 2016 147 pm 20th feb 2017 in short the process of a calorimeter involves measuring the heat of a fuel sample when burned under stable temperature conditions to evaluate the heating energy of the fuel sample. Temperature of the water. A bomb calorimeter is a device that measures the heat given off or taken in by a reaction.
In the human body the reaction takes place over a series of many intermediate reactions gradually releasing energy along the way. The rise in temperature of the water is recorded and used to calculate the amount of heat produced.
 Constant Volume Calorimetry Chemistry Libretexts
Constant Volume Calorimetry Chemistry Libretexts
 Chemistry Past Exams Thermochemistry 2010
Chemistry Past Exams Thermochemistry 2010
 Which Diagram Is A Bomb Calorimeter Pretty Aqa Biology A Level Bomb
Which Diagram Is A Bomb Calorimeter Pretty Aqa Biology A Level Bomb
 Bomb Calorimeter Chemistry Dictionary Glossary
Bomb Calorimeter Chemistry Dictionary Glossary
 Experiment 1 Adiabatic Bomb Calorimeter
Experiment 1 Adiabatic Bomb Calorimeter
 File Bomb Calorimeter Diagram Png Wikimedia Commons
File Bomb Calorimeter Diagram Png Wikimedia Commons
 Me 354 Lab Bomb Calorimeter Experiment
Me 354 Lab Bomb Calorimeter Experiment
 Bomb Calorimetry Heat Of Combustion Of Naphthalene
Bomb Calorimetry Heat Of Combustion Of Naphthalene
 How Does A Bomb Calorimeter Work
How Does A Bomb Calorimeter Work
 Enthalpy Of Combustion Bomb Calorimeter
Enthalpy Of Combustion Bomb Calorimeter

0 Response to "Which Diagram Is A Bomb Calorimeter"
Post a Comment