How To Calculate Bond Order From Mo Diagram
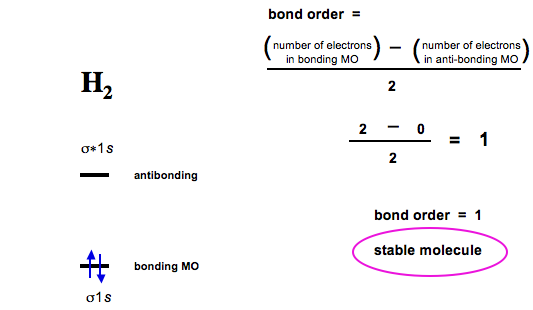
In molecular orbital diagram we just need to calculate the number of electrons in anti bonding orbital and bonding orbital then we can use the formula in order to calculate bond order is. For example o2 has a double bond and has a bond order of 2.
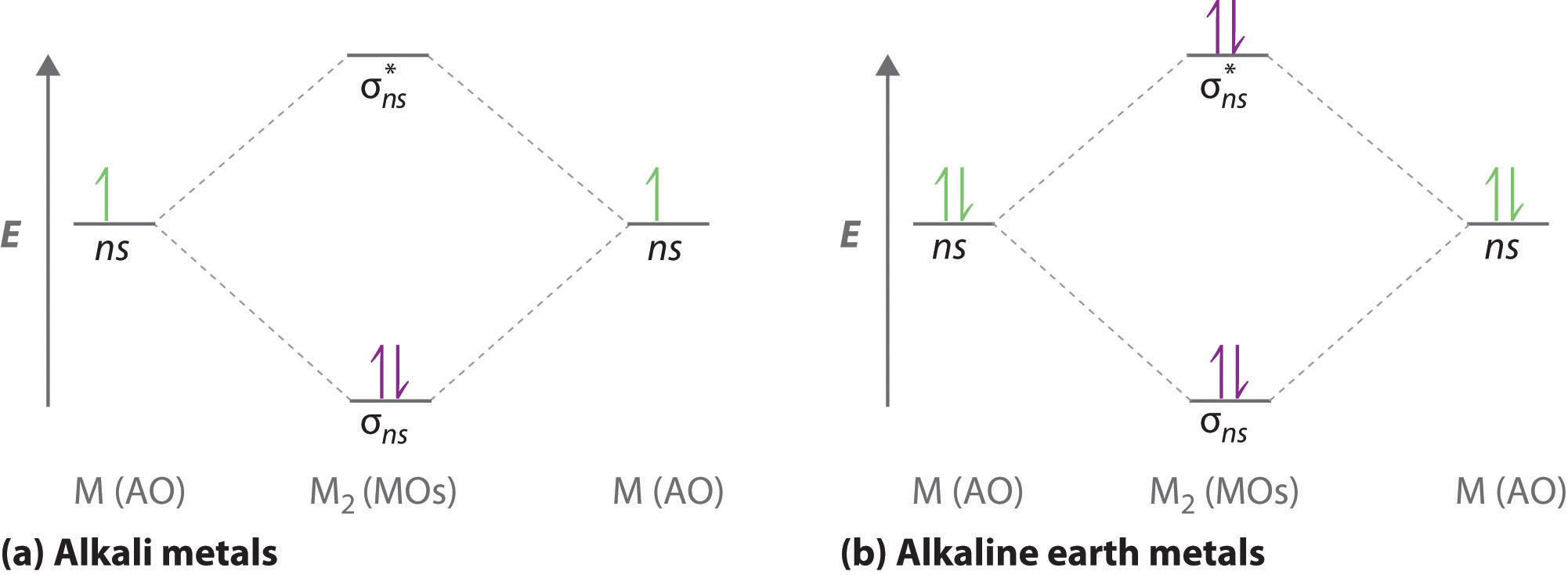 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
For instance the bond order of diatomic nitrogen nn is 3 and bond order between the carbon atoms in h hc h is also three.
How to calculate bond order from mo diagram. In its most basic form the bond order is the number of bonded electron pairs that hold two atoms together. The bond order in sulfur dioxide for example is 15 the average of an s o single bond in one lewis structure and an so double bond in the other. The bond order describes the stability of the bondthe molecular orbital provides an easy understanding of the concept.
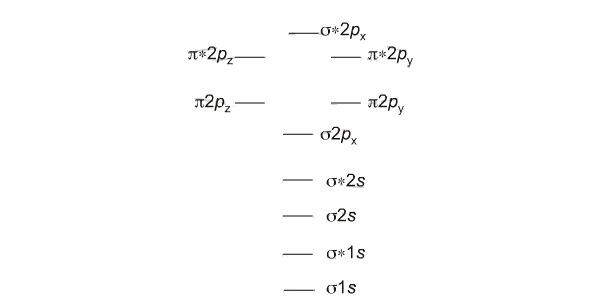
In diatomic nitrogen nn for example the bond order is 3 while in acetylene hcch the bond order between the two carbon atoms is 3 and the ch bond order is 1. 3a1 is the sigma2pz bonding mo but its relatively nonbonding with respect to oxygen. Basically you can calculate the bond order by counting the bonds with the help of the lewis structure.
1b2 is the pi2py bonding mo. A double covalent bond a bond order of two. If we draw the lewis structure for no3 we may find out that its a resonance structure with one double bond and two single bonds.
A triple covalent bond three and so on. Bond order no. 2b2 is the pi2py antibonding mo.
Bond order indicates the stability of a bond. The bond order shows the number of chemical bonds present between a pair of atoms. 1b1 is the pi2px bonding mo.
1a1 is the sigma2s bonding mo. In molecular orbital theory we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital cancel the effect of one bond. Bond order is the number of chemical bonds between a pair of atoms.
So the bond order is 4. Of electrons in anti bonding mo no. A single covalent bond has a bond order of one.
2b1 is the pi2px antibonding mo. Quick overview of what the labels correspond to what mos. Consider how atoms come together into molecules.
Of electrons in bonding mo 2. 2a1 is the sigma2s antibonding mo. 4a1 is the sigma2pz antibonding mo.
 How To Build Molecular Orbitals Chemistry Libretexts
How To Build Molecular Orbitals Chemistry Libretexts
 Molecular Orbital Theory Boundless Chemistry
Molecular Orbital Theory Boundless Chemistry
Molecular Orbital Diagram For H2 Michaelhannan Co
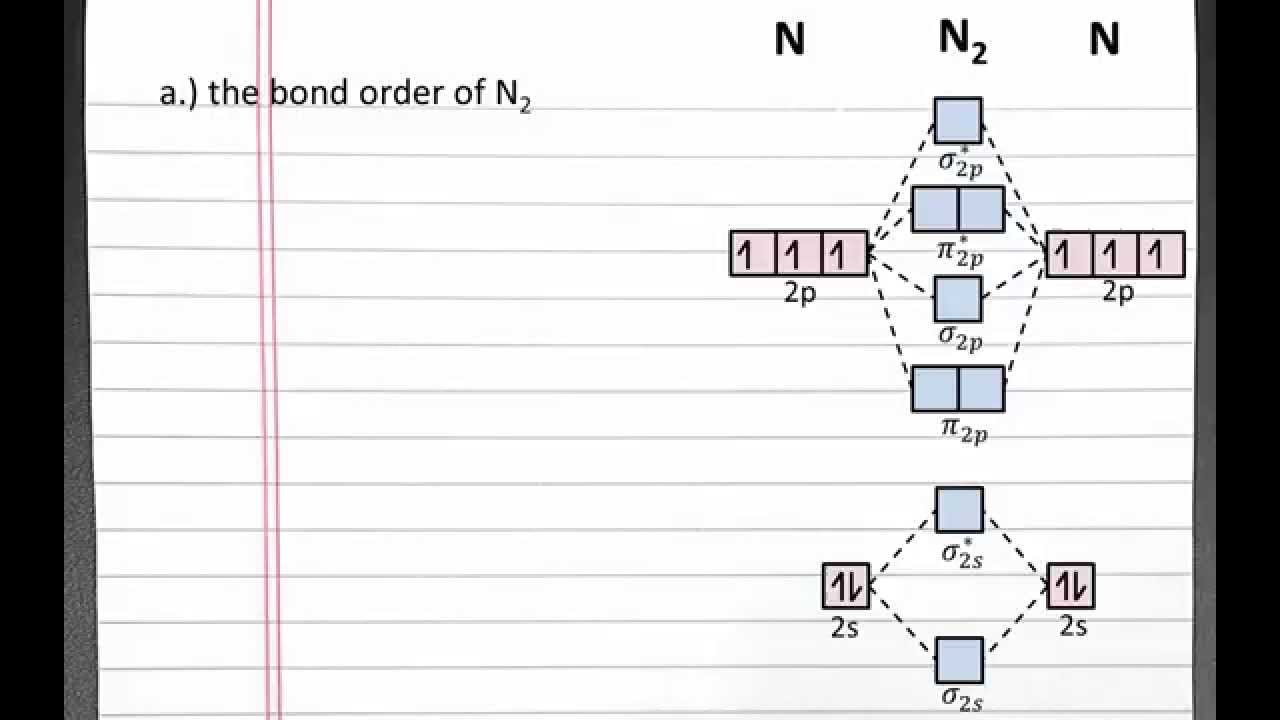 Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
Chemistry 101 Molecular Orbital Theory Bond Order Bond Strength
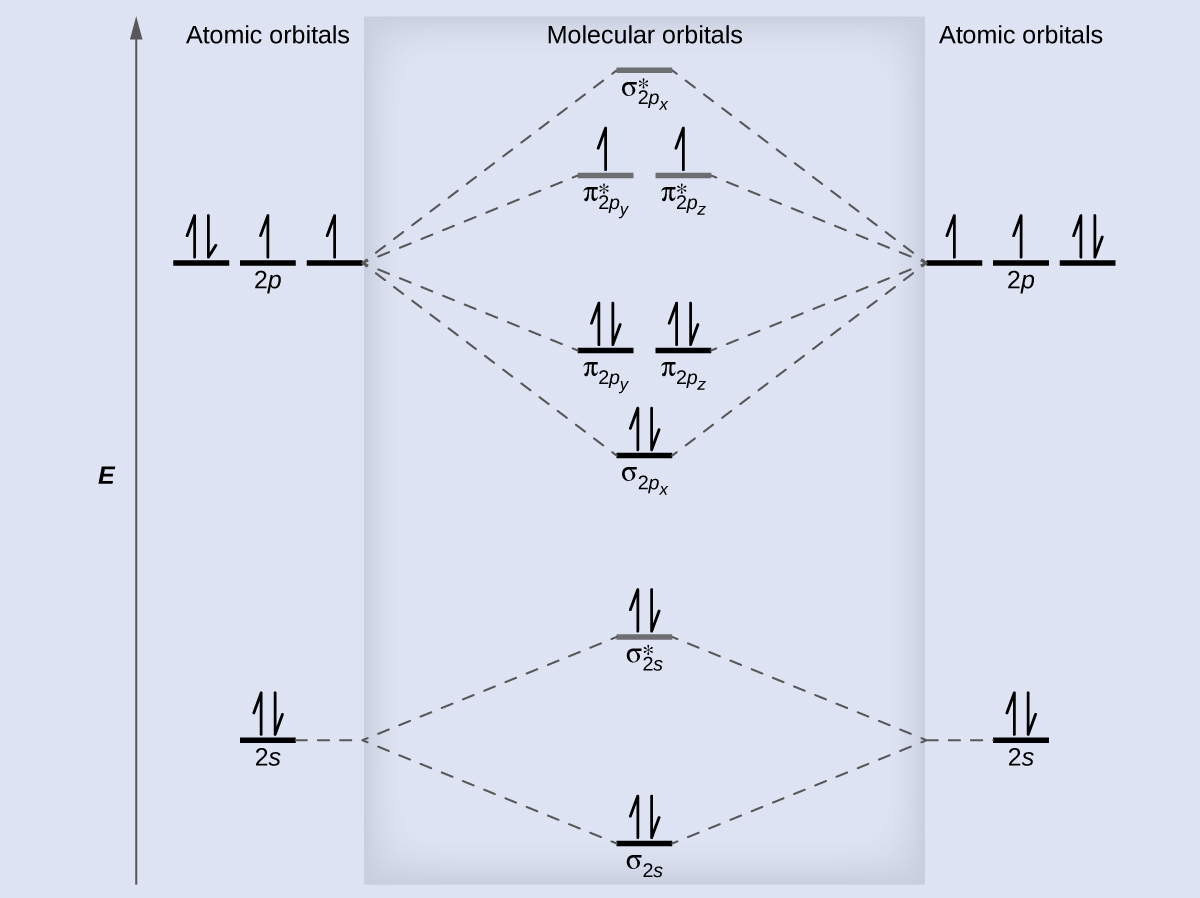 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
Molecular Orbital Diagram Wikipedia
 Molecular Orbital Theory Iii Bond Order And Stability Youtube
Molecular Orbital Theory Iii Bond Order And Stability Youtube
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Sparknotes Organic Chemistry Orbitals Problems Molecular Orbital
Molecular Orbital Theory Chemistry Encyclopedia Structure
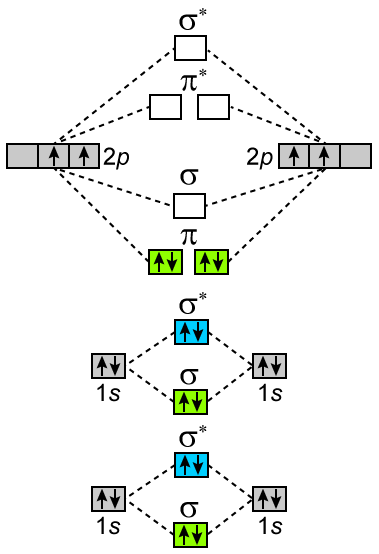 Write Molecular Orbital Configuration Of C2 Predict Magnetic
Write Molecular Orbital Configuration Of C2 Predict Magnetic
M O Diagram For B2 Chemistry Community
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
Introduction To Molecular Orbital Theory
 Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
0 Response to "How To Calculate Bond Order From Mo Diagram"
Post a Comment