Does This Diagram Represent An Increase Or Decrease In The Internal Energy Of The System
A does this diagram represent an increase or decrease in the internal energy of the system. Increase decrease what sign i.
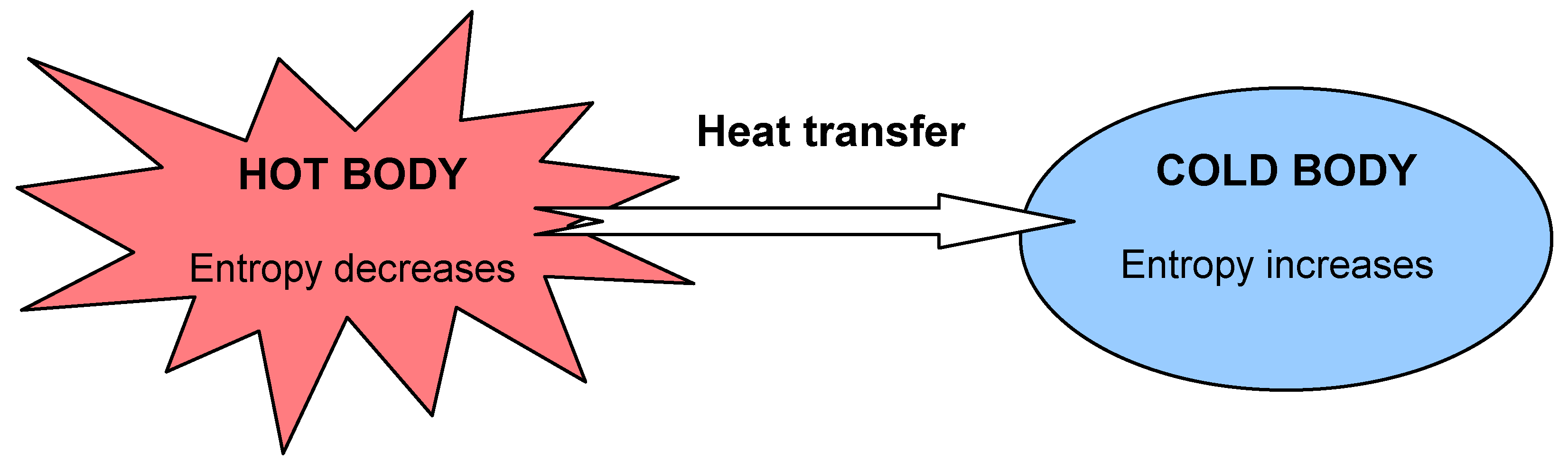 Entropy Free Full Text Energy Entropy And Exergy Concepts And
Entropy Free Full Text Energy Entropy And Exergy Concepts And
B what sign is given to e for this process.
Does this diagram represent an increase or decrease in the internal energy of the system. Originproblemid1097921descriptionconsider the energy diagram shown in the from chemistry 151 at rio salado community college. C if there is no work associated with the process is it exothermic or endothermic. B what sign is given to e for this process.
If work is done by a system will the internal energy of the system increase or decrease. C if there is no work associated with the process is it exothermic or endothermic. Calculate delta e energy and determine whether the process is endothermic or exothermic for the follwing cases.
Consider the accompanying energy diagram. B q 150 kj and w 657 j. If work is done by a system does the internal energy of the system increase or decrease.
They will both decrease. Chapter 24 thermodynamics review. 53 consider the accompanying energy diagram.
C the system releases 575 kj of heat while doing 225 kj of work on the surroundings. If work is done on a system does the internal energy of the system increase or decrease. Diagram represent an increase of internal energy of the system e eproduct ereactant eproduct is bigger than ereactant so e is positive if no work is associated with the process the reaction is.
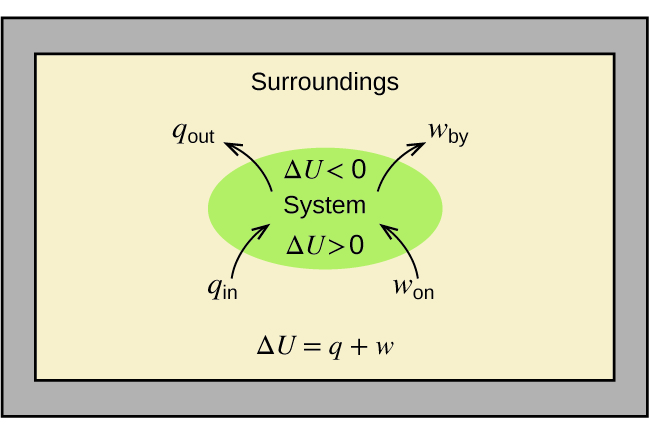
Incorrectly answering a question in a part or hint. The first law of thermodynamics energy cannot be created nor destroyed but it can be transferred between a system and its surroundings. Thermodynamics reading check and hw.
A a system absorbs 105 kj of heat from its surroundings while doing 29 kj of work on the surroundings. Bike pump use warms up. Answer to does this diagram represent an increase or decrease in the internal energy of the system.
The change in internal energy δetexttip delta e delta e is positive if the system absorbs energy and it is negative if the system releases energy. A does this diagram represent an increase or decrease in the internal energy of the system. If work is done adiabatically on a system will the internal energy of the system increase or decrease.

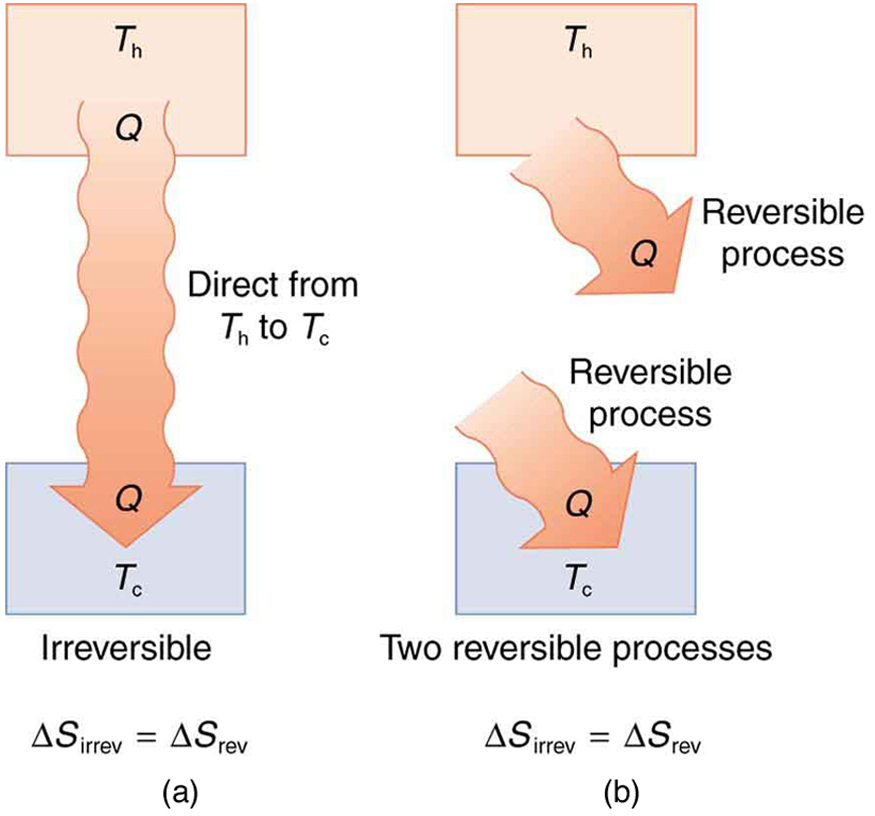 Entropy And The Second Law Of Thermodynamics Disorder And The
Entropy And The Second Law Of Thermodynamics Disorder And The
Energy Enthalpy And The First Law Of Thermodynamics
The First Law Of Thermodynamics
 Data Flow Diagram Symbols Types And Tips Lucidchart
Data Flow Diagram Symbols Types And Tips Lucidchart
 Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
 Solved Does This Diagram Represent An Increase Or Decreas
Solved Does This Diagram Represent An Increase Or Decreas
 Chapter 3c The First Law Closed Systems Diesel Cycle Engines
Chapter 3c The First Law Closed Systems Diesel Cycle Engines
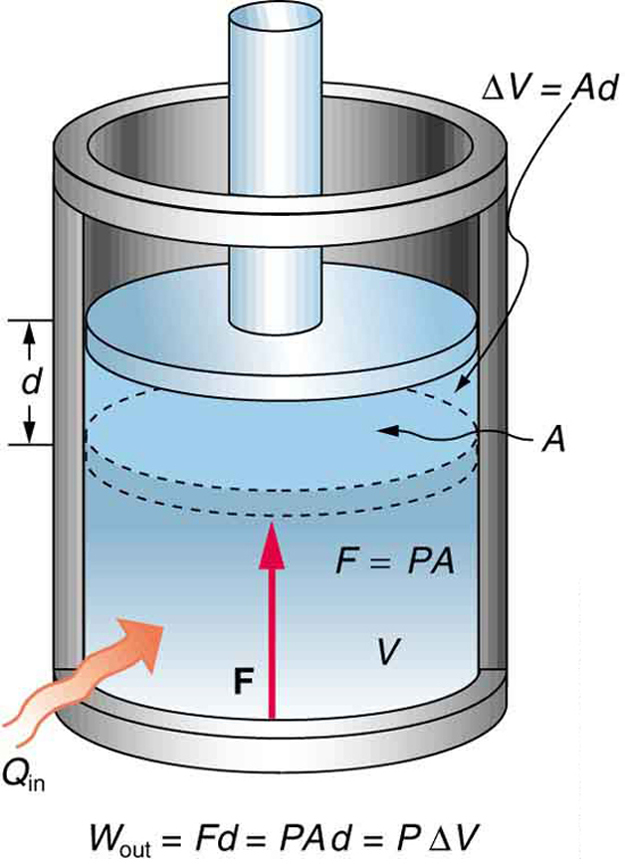 The First Law Of Thermodynamics Boundless Physics
The First Law Of Thermodynamics Boundless Physics
Lecture Notes On Thermodynamics
 What S The Basic Difference Between Entropy And Enthalpy Quora
What S The Basic Difference Between Entropy And Enthalpy Quora

Chemistry The Central Science Chapter 5 Section 2
 Thermodynamics Internal Energy Of A System Can Be Increased Either
Thermodynamics Internal Energy Of A System Can Be Increased Either
The First Law Of Thermodynamics
Energy Enthalpy And The First Law Of Thermodynamics
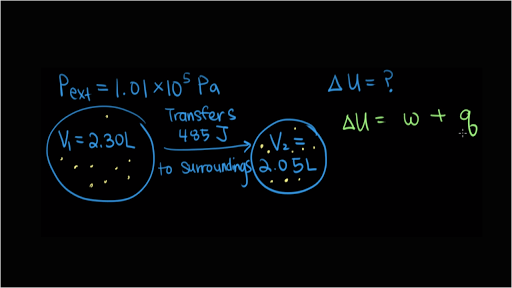
0 Response to "Does This Diagram Represent An Increase Or Decrease In The Internal Energy Of The System"
Post a Comment