Energy Diagram For Exothermic Reaction
You can start with a generic potential energy diagram for an exothermic reaction. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions.
 The Sn2 Mechanism Energy Diagram Mechanism And Stereochemisy
The Sn2 Mechanism Energy Diagram Mechanism And Stereochemisy
Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.
Energy diagram for exothermic reaction. The extra energy is released to the surroundings. A reaction is defined as exothermic if you put in less energy to break the bonds of the reactants the is the activation energy than it is released when the products are formed. Energy profile diagrams for endothermic and exothermic reactions.
Energy reactants products exothermic reactions the reactants have more potential energy than the products have. There is a greater difference in energy between the reactants and products. Energy diagrams for endothermic and exothermic reactions.
The green arrow is longer. The energy values points on the hyper surface along the reaction coordinate result in a 1 d energy surface a line and when plotted against the reaction coordinate energy vs reaction coordinate gives what is called a reaction coordinate diagram or energy profile. More on pe diagrams.
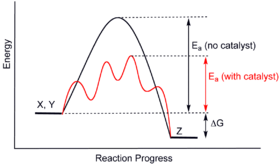
A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. Heat of reaction is given the symbol ah and is usually measured in kilojoules kj. So the activation energy is the minimum amount of energy required for a reaction to take place.
Energy must be input in order to raise the particles up to the higher energy level. Endothermic reactions take in energy and the temperature of the surroundings decreases. δh total energy content of products total energy content of reactants h products h.
The reaction shown by the second diagram is more exothermic. It also shows the effect of a catalyst on the forward and reverse activation energy. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below.
An energy level diagram shows whether a reaction is exothermic or endothermic. In other words the products are less stable than the reactants. Reactants products energy.
The amount of heat energy released or absorbed during a chemical reaction is called the heat of reaction.
Reaction Energy Profiles Activation Energy Exothermic Endothermic
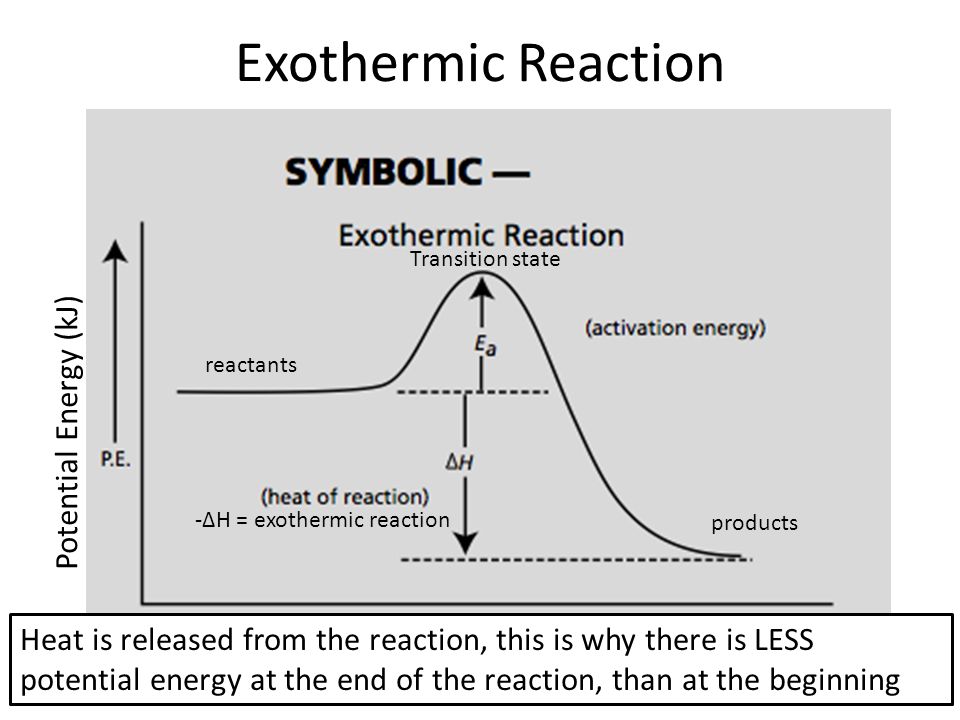 Endothermic And Exothermic Reactions Ppt Video Online Download
Endothermic And Exothermic Reactions Ppt Video Online Download
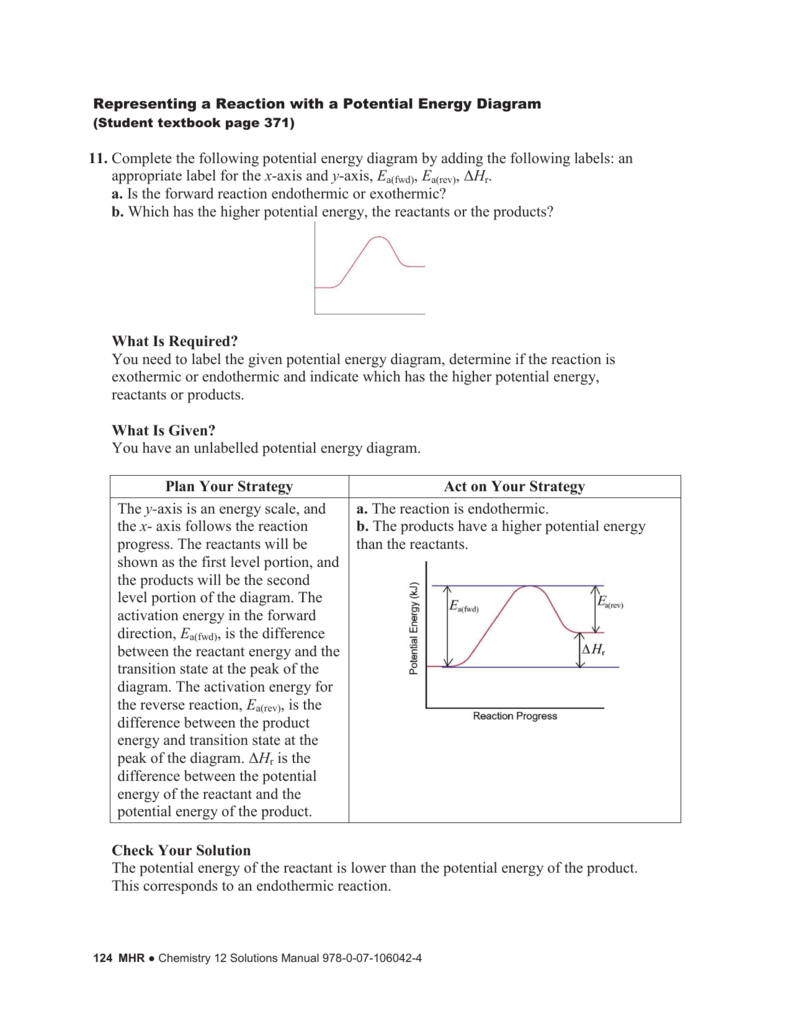 Representing A Reaction With A Potential Energy Diagram
Representing A Reaction With A Potential Energy Diagram
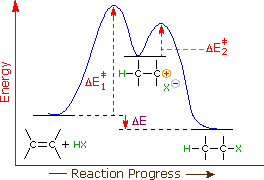 Endothermic Energy Diagram Energy Etfs
Endothermic Energy Diagram Energy Etfs
 Why Do All Metabolic Reactions Release Heat Such As Atp Hydrolysis
Why Do All Metabolic Reactions Release Heat Such As Atp Hydrolysis

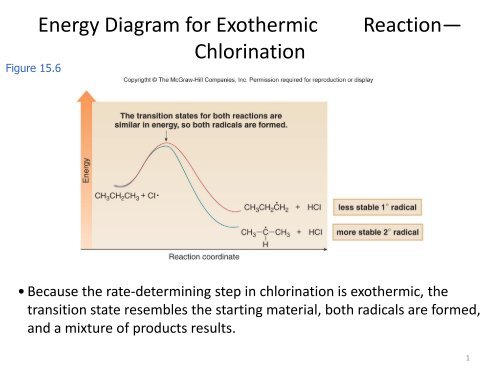 Energy Diagram For Exothermic Reaction Chlorination
Energy Diagram For Exothermic Reaction Chlorination
 Endothermic And Exothermic Reactions Mcat Physical
Endothermic And Exothermic Reactions Mcat Physical
Comparing Endothermic And Exothermic Potential Energy Diagrams
Exothermic Reaction Energy Diagram The Flow Of Energy Heat Air
 Energy Changes In Chemical Reactions Energy And Chemical Change
Energy Changes In Chemical Reactions Energy And Chemical Change
 Solved 20 Value 4 00 Points 1 Attempts Left Check My Wor
Solved 20 Value 4 00 Points 1 Attempts Left Check My Wor
 Potential Energy Diagram For An Exothermic Reaction Cbsquared
Potential Energy Diagram For An Exothermic Reaction Cbsquared
 Drawing Energy Diagrams Exothermic Endothermic Catalysts Youtube
Drawing Energy Diagrams Exothermic Endothermic Catalysts Youtube
 Endothermic And Exothermic Reactions Ap Biology Chemistry Ap
Endothermic And Exothermic Reactions Ap Biology Chemistry Ap
Endothermic Reaction Energy Diagram Cute Energy Diagram Endothermic
Exothermic Reaction Energy Diagram Materials Free Full Text Air
 Potential Energy Diagrams Chemistry Catalyst Endothermic
Potential Energy Diagrams Chemistry Catalyst Endothermic
Endothermic Versus Exothermic Reactions
 Elegant Of Exothermic Energy Diagram Potential Diagrams Read
Elegant Of Exothermic Energy Diagram Potential Diagrams Read
Potential Energy Diagram Of Exothermic Reaction Nemetas
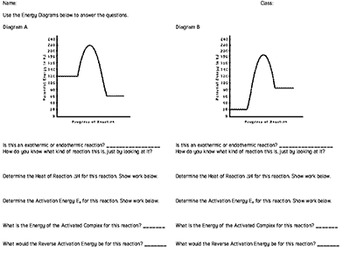 Potential Energy Diagram Practice Endothermic And Exothermic Reactions
Potential Energy Diagram Practice Endothermic And Exothermic Reactions
0 Response to "Energy Diagram For Exothermic Reaction"
Post a Comment