Orbital Diagram For F
The aufbau principle the pauli exclusion principle and hunds rule. Since 1s can only hold two electrons the next 2 electrons for f go in the 2s orbital.
 The Molecular Orbital Diagram Of Fluorine Youtube Youtube
The Molecular Orbital Diagram Of Fluorine Youtube Youtube
In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital.
Orbital diagram for f. Orbital diagrams are pictorial descriptions of the electrons in an atom. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao method in particular. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.
These names together with the value of n are used to describe the electron configurations of atoms. 1s22s22p63s23p64s23d4 the orbital diagram above is formatted in such a manner as to place the various orbital types at different energy levels. The electron configuration for chromium is.
Orbital diagrams are a visual way to show where the electrons are located within an atom. The remaining five electrons will go in the 2p orbital. In addition to listing the principle quantum number n and the subshell ell the orbital diagram shows all the different orientations and the spin of every electron.
The simple names s orbital p orbital d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ 0 1 2 and 3 respectively. An orbital diagram naturally leads to the writing of an electron configuration. Orbital diagrams and electron configurations.
Orbital diagrams must follow 3 rules. According to the auf bau principle each electron occupies the lowest energy orbital. The pauli exclusion principle says that only two electrons can fit into an single orbital.
Therefore the f electron configuration will be 1s 2 2s 2 2p 5. Many times it is necessary to see all the quantum numbers in an electron configuration this the purpose of the orbital diagram. Three rules are useful in forming orbital diagrams.
Explain how the quantum mechanical model is based upon the idea that electrons travel in waves. Define orbital electron cloud. 321 describe how schrodinger advanced the understanding of atomic structure.
In an orbital filling diagram the individual orbitals are shown as circles or squares and orbitals within a sublevel are drawn next to each other horizontally.
F Block Orbital Diagram Wiring Diagram
 What Is The Electron Configuration Of F Socratic
What Is The Electron Configuration Of F Socratic
Orbital Diagram For F New Orbital Energy Diagram Fluorine Trusted
What Is An Orbital Diagram Orbital Diagram For Fluorine Awesome 0d
The Diagram F Orbital Molecular For Oxygen O2 Oasissolutions Co
Wave Quantum Mechanic Theory F Orbitals Orbital Diagram Atomic For
 Orbital Diagram Of The Cu Gd System Resulting From Unrestricted
Orbital Diagram Of The Cu Gd System Resulting From Unrestricted
Orbital Diagram For Fluorine Fresh Orbital Diagram Fluorine
Sparknotes Molecular Orbitals Molecular Orbital Theory
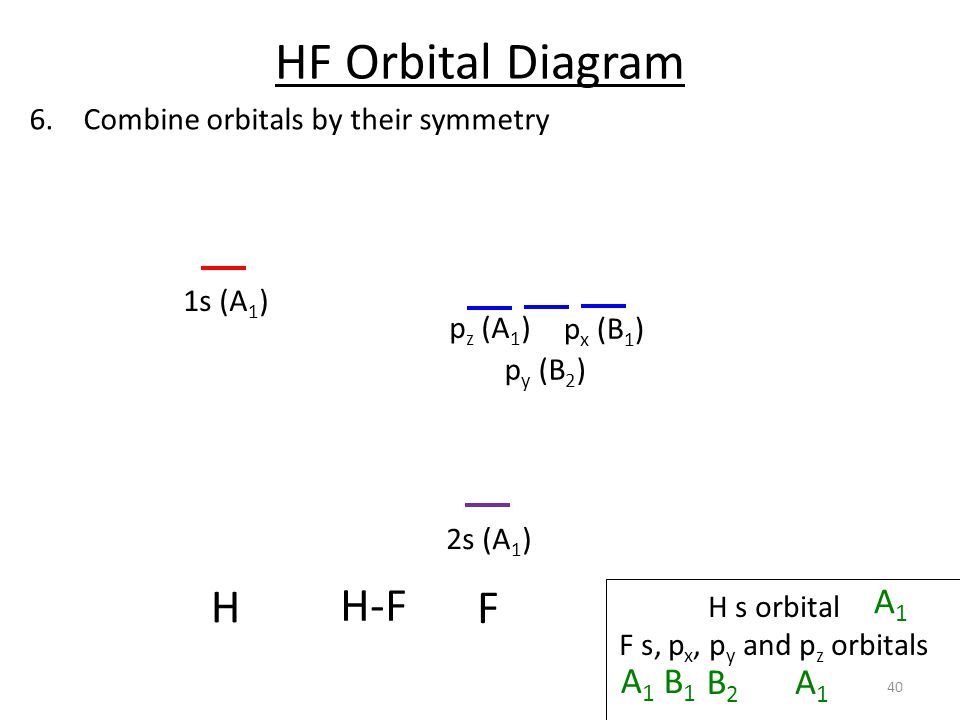 Part 2 7 Orbital Diagrams Ppt Download
Part 2 7 Orbital Diagrams Ppt Download
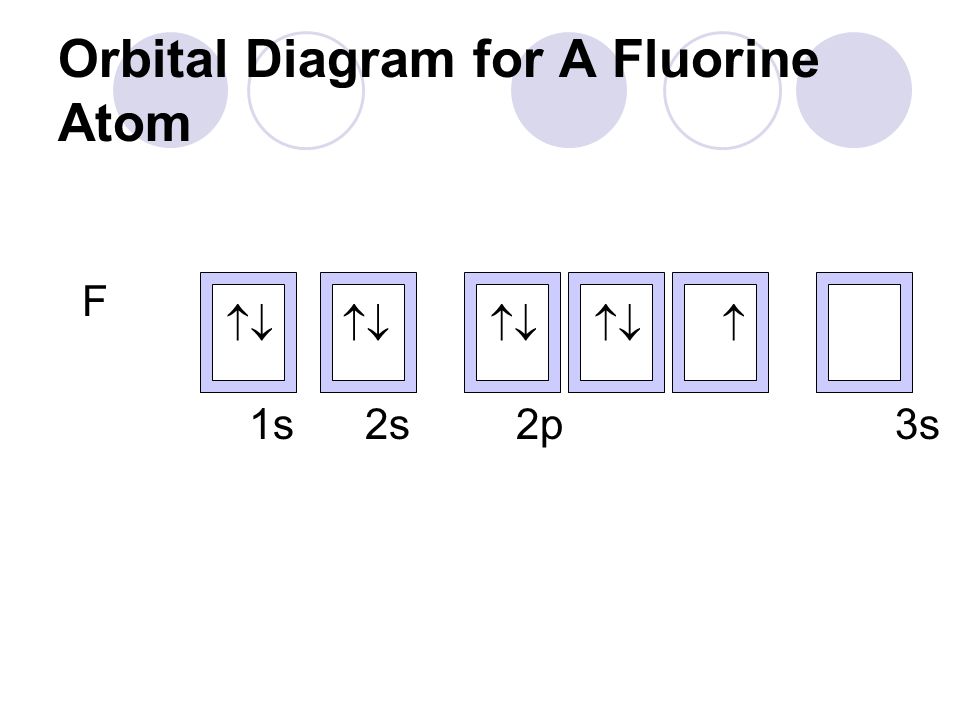 The Atom Ppt Video Online Download
The Atom Ppt Video Online Download
Orbital Diagram For Fluorine Air American Samoa
Molecular Orbitals In Hydrogen Fluoride
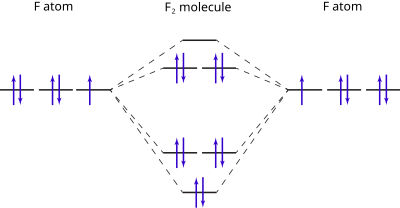 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
Section 5 Observation 3 Ionization Energies Of Diatomic Molecule
Diagram F Orbital Diagram 5 Linear F Orbital Diagram
 Electron Configuration Boundless Chemistry
Electron Configuration Boundless Chemistry
 Inorganic Chemistry Understanding Fhf Molecular Orbital Diagram
Inorganic Chemistry Understanding Fhf Molecular Orbital Diagram
Molecular Orbitals For Heterogeneous Diatomic Molecules
0 Response to "Orbital Diagram For F"
Post a Comment