Electron Dot Diagram For Fluorine
Lithium is the most stable element because it has to lose only one electron to achieve a stable configuration. Were going to do the lewis structure for f2 fluorine gas.
And well start looking on the periodic table.
Electron dot diagram for fluorine. There are 7 valence electrons in fluorine or any halogen for that matter. Lewis electron dot diagrams for ions have less for cations or more for anions dots than the corresponding atom. 1 answer anor277 jan 7 2017.
Choose the statement that correctly identifies the most stable of the elements. Fluorine is in group 7 or sometimes called 17 and that means that it will have 7 valence electrons. Fluorine is in group 7 sometimes called group vii or group 17.
After that i draw the lewis dot structure for fluorine f. And thus the neutral atom has 7 valence electrons. What is the lewis electron dot diagram for a fluoride ion.
Choose the statement the correctly identifies the most stable of the elements. Carbon is the most stable element because it can form four. Once it reacts with a nonmetal to form fluoride fluorine with a negative 1 charge you can put the 8th electron on this diagram stability comes from filling up the valence electrons.
Chemistry covalent bonds drawing lewis structures. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1.
Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1. A yellow extremely reactive gas. With the next element sodium.
Fluorine is in group 17 of the periodic table. Socratic meta featured answers chemistry. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
Lewis structure electron dot diagram for hydrogen fluoride or the 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom which may or may not be circled are referred to as a covalent bond or a single covalent bond. Fluorine and neon have seven and eight dots respectively. Since it is in group 7 it will have 7 valence electrons.
The number of dots equals the number of valence electrons in the atom.
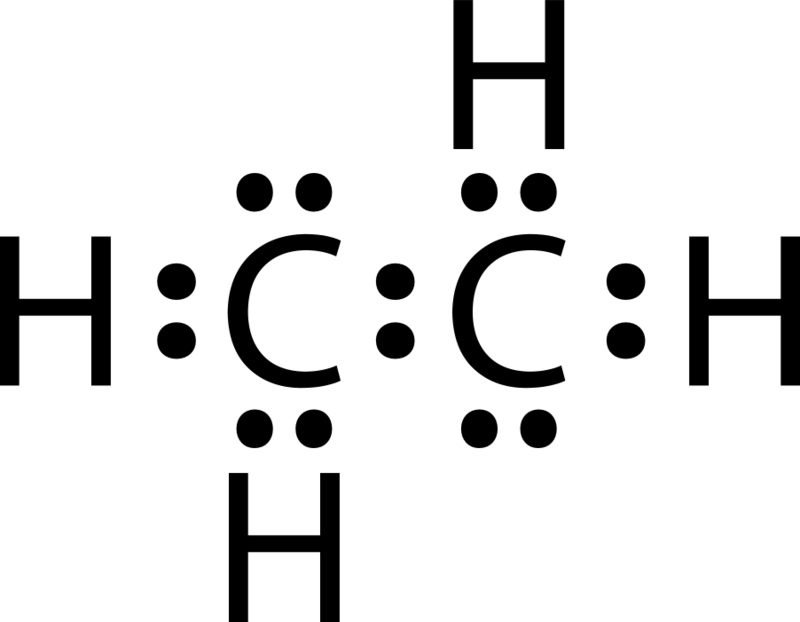
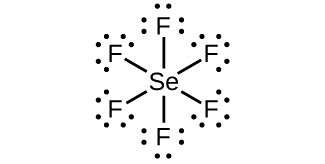 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
Electron Orbital Diagram Fluorine Dot Diagram Daytonva150
Fluorine Electron Dot Diagram 57298 Usbdata
Ap Chemistry Summer Assignment
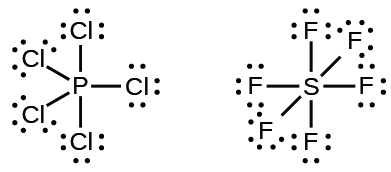 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry

Fluorine Dot Diagram Beautiful Radon Periodic Table Lovely Radon
Aluminum Chloride Lewis Dot Structure For Aluminum Chloride
Electron Dot Diagram For Potassium Michaelhannan Co

Diagram Fluorine Dot Diagram Images For S Structure Of Fluorine
 Which Is The Correct Electron Dot Structure For The Element Fluorine
Which Is The Correct Electron Dot Structure For The Element Fluorine
Fluorine Electron Dot Diagram Best Of Itext Chapter 5 Section 3
Bonding Basics 19 728 Jpg Cb 1262641352 Fluorine Lewis Dot Structure
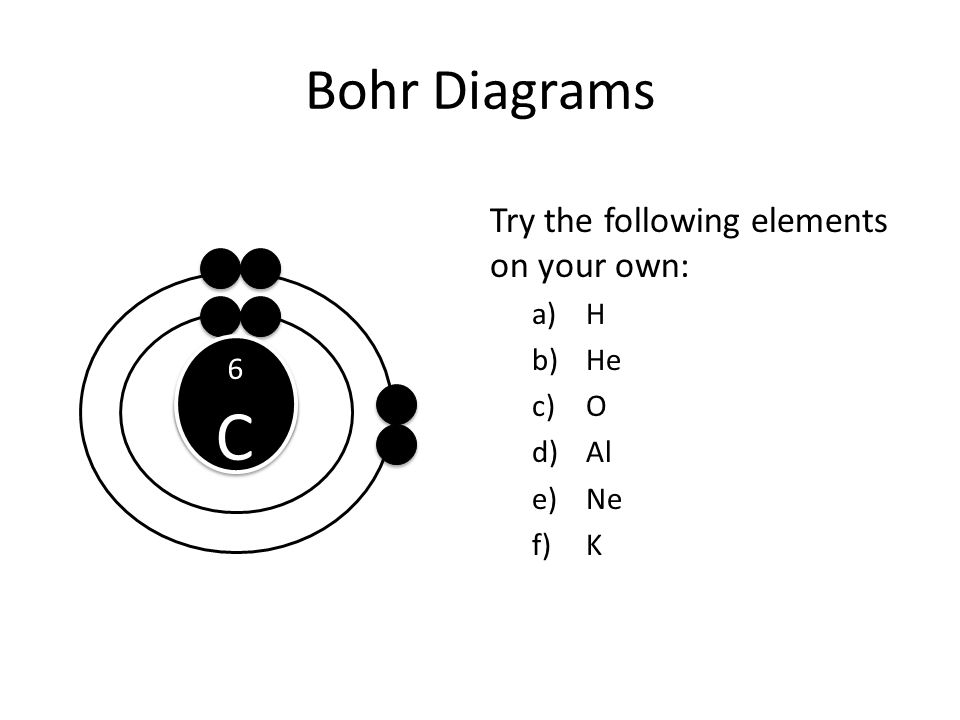 Bohr Diagrams Compu Ibmdatamanagement Co
Bohr Diagrams Compu Ibmdatamanagement Co
Valence Shell Electron Pair Repulsion
 Fluorine Lewis Dot Structure Luxury Exceptions To The Octet Rule
Fluorine Lewis Dot Structure Luxury Exceptions To The Octet Rule
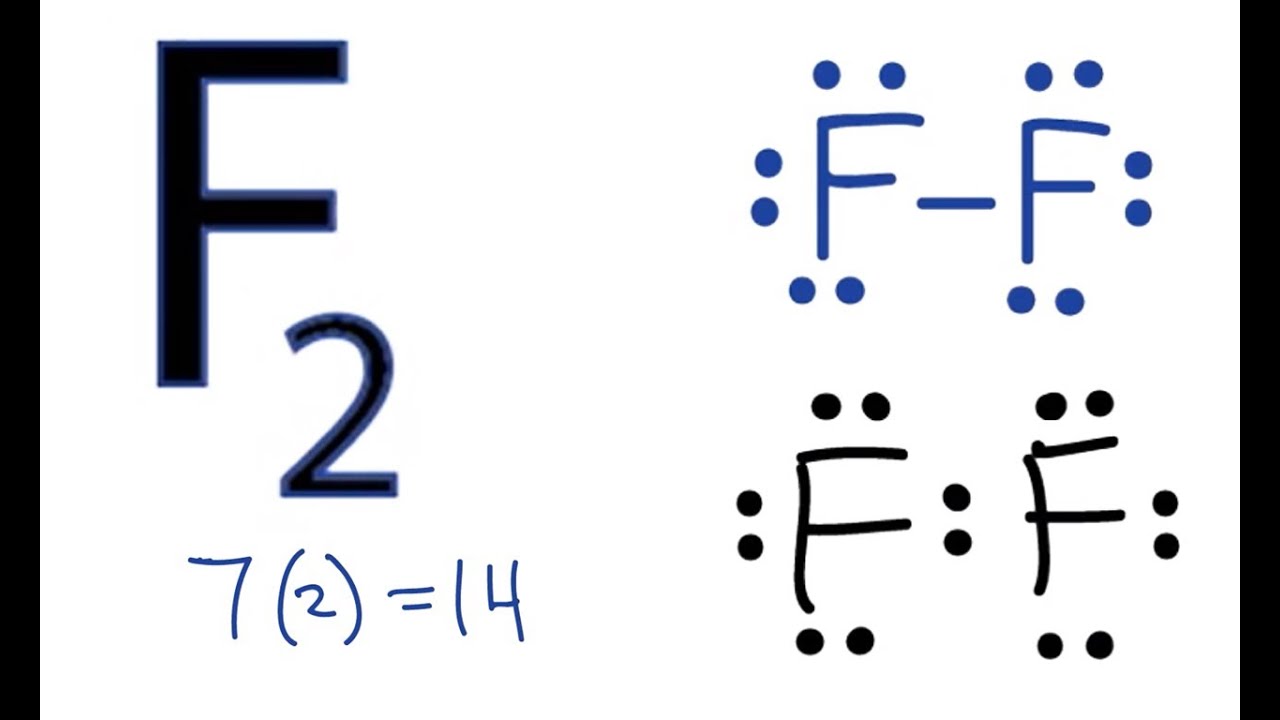


0 Response to "Electron Dot Diagram For Fluorine"
Post a Comment