Which Statement Is True Of The Atom Shown In The Diagram
Jones says an atom has 3 electrons in the first shell and four electrons in the second shell. When elements react their atoms combine in simple whole number ratios.
 Structures Of Nalidixic Acid And Some Of The Fluoroquinolones
Structures Of Nalidixic Acid And Some Of The Fluoroquinolones
Chapters 2 and 3 study guide chapters 2 and 3 the mass.
Which statement is true of the atom shown in the diagram. N 4 is sp3 hybridized. Which statement is true of the energy levels of electrons in shells. Which of the following statements is true regarding the structure shown.
By making two covalent bonds an o atom with 8 protons fills its valence shell. C 2 and o 6 are sp2 hybridized. Designate the atom shown in question 6 in the form name of element mass.
Another isotope of the same element might have. An atom has too many electrons. Radioactive decay is likely to occur when.
Mass number 16 atomic number 7. 2 it must occur prior the mechanical digestion of food in the oral cavity. The picture shows one big red one in the middle with one blue ring on the outside that had 2 smaller red ones and another blue ring outside of the first blue ring that had 8 smaller red ones.
Which is a correct statement about the process shown in the diagram. The second shell cant have 4 electrons. The chemical basis of life.
The valence shell has higher energy than other occupied shells. C 2 o 6 and n 4 are all sp2 hybridized d. All of the above.
Two electrons fill the first shell and 5 go into the second valence shell. The only sp2 hybridized atom is c 2 b. Which of the following is true about the atom shown choose two that apply.
All atoms of the same element are identical. None of the above. Circle the letter of each statement that is true about the average atomic mass.
All of the above. The neutral atom has 7 electrons. No shell can hold more than 2 electrons.
B electrons must lose energy to move from the first to the second shell. Someone should tell dr. All matter consists of tiny particles called atoms.
1 it transports nutrients within the digestive tract. An atom has 8 protons 8 neutrons and 8 electrons. All the electrons in an atom have similar amounts of energy.
The atom needs 3 more electrons to fill the valence shell. Around the 1870s this scientist studied mysterious rays in sealed glass tubes. The only sp3 hybridized atoms are c 1 c 3 and c 5 c.
Which of the following statements is true regarding the structure shown. The first shell must fill before the second shell can have electrons. The second shell should have 8 electrons.
3 it emulsifies fats for hydrolysis in the small intestine 4 it increases water absorption by the esophagus this is the picture. The diagram represents a portion of the esophagus. 5 atomic structure and the periodic table atoms they did not explain chemical behavior and they lacked experimental support.
 8 5 Atomic Spectra And X Rays Physics Libretexts
8 5 Atomic Spectra And X Rays Physics Libretexts
 5 2 Hybrid Atomic Orbitals Chemistry Libretexts
5 2 Hybrid Atomic Orbitals Chemistry Libretexts
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 A New Model Of The Atom Wikibooks Open Books For An Open World
A New Model Of The Atom Wikibooks Open Books For An Open World
 Solved Questlon 8 Objective Knowledge Check Complete The
Solved Questlon 8 Objective Knowledge Check Complete The
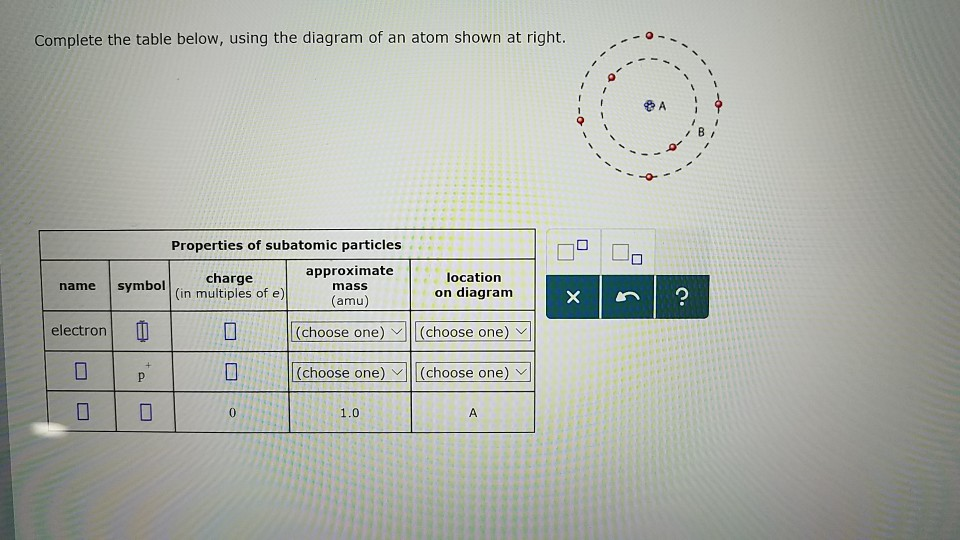
 Solved Complete The Table Below Using The Diagram Of An
Solved Complete The Table Below Using The Diagram Of An
 The Atom Diagram Isn T What An Atom Looks Like
The Atom Diagram Isn T What An Atom Looks Like
 5 2 Lewis Diagrams Chemistry Libretexts
5 2 Lewis Diagrams Chemistry Libretexts
 Atom Diagrams Electron Configurations Of The Elements
Atom Diagrams Electron Configurations Of The Elements
 Models Of The Atom The Atom Siyavula
Models Of The Atom The Atom Siyavula
 The Atom Diagram Isn T What An Atom Looks Like
The Atom Diagram Isn T What An Atom Looks Like
 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 Thomson Atomic Model Description Image Britannica Com
Thomson Atomic Model Description Image Britannica Com
 High School Chemistry Atomic Terminology Wikibooks Open Books For
High School Chemistry Atomic Terminology Wikibooks Open Books For
 Molecular Structure Of The R Enantiomer Of 4b The Only Hydrogen
Molecular Structure Of The R Enantiomer Of 4b The Only Hydrogen
 Solved Sort Each Of The Items Into The Appropriate Bin Us
Solved Sort Each Of The Items Into The Appropriate Bin Us

0 Response to "Which Statement Is True Of The Atom Shown In The Diagram"
Post a Comment