Consider The Following Reaction Energy Diagram
This state is also known as an activated complex. The activation energy for the formation of hi is 167 kj.
Home study science chemistry chemistry questions and answers consider the following reaction energy diagram.
Consider the following reaction energy diagram. The number of molecules having e r or greater doubles. The activation energy in the diagram is 438 kcal mole letter c. At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products.
Draw a rough sketch of the energy profile for each. Choose all that apply 1 the reaction is exothermic 2 the reacting molecules must collide with the correct orientation 3 heat is released 4 the reactants have a higher potential energy than the products 5 the reactants have a lower potential energy than the products 6 activation energy must. How many elementary steps are in the reaction.
How many elementary steps are in the reaction me. ½ h 2g ½ i 2g hi g h 28 kj. Consider the following energy diagram showing the.
From the diagram when the temperature of reactants are raised from 20c to 30c. For the following reaction profile indicatea. Reaction a in endothermic and reaction b is exothermic.
In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Therefore the spike after h2 and i2 is reacted is the activation energy of the reaction. The activation energy for the following reaction is 125 kjmol and δe for the reaction is 216 kjmol.
You have to note that activation energy is the energy needed for the reaction to occur and produce products. Reaction a is exothermic and reaction b is endothermic. Does this provide evidence that a chemical reaction took place.
The activation energy for the following reaction is 125 kjmol and δe for the reaction is 216 kjmol. Consider the following reaction energy diagram. Consider the following reaction.
Yes because energy is released consider the energy diagram for a chemical reaction in figure 6 3. Consider two gases a and b that are in a container at room temperature. Which of the following is true about the potential energy diagram for 2h2 o2 2 h2o h 4866 kj.
 Reaction Mechanism Britannica Com
Reaction Mechanism Britannica Com
 Gen Chem Discuss Chemical Equilibrium
Gen Chem Discuss Chemical Equilibrium
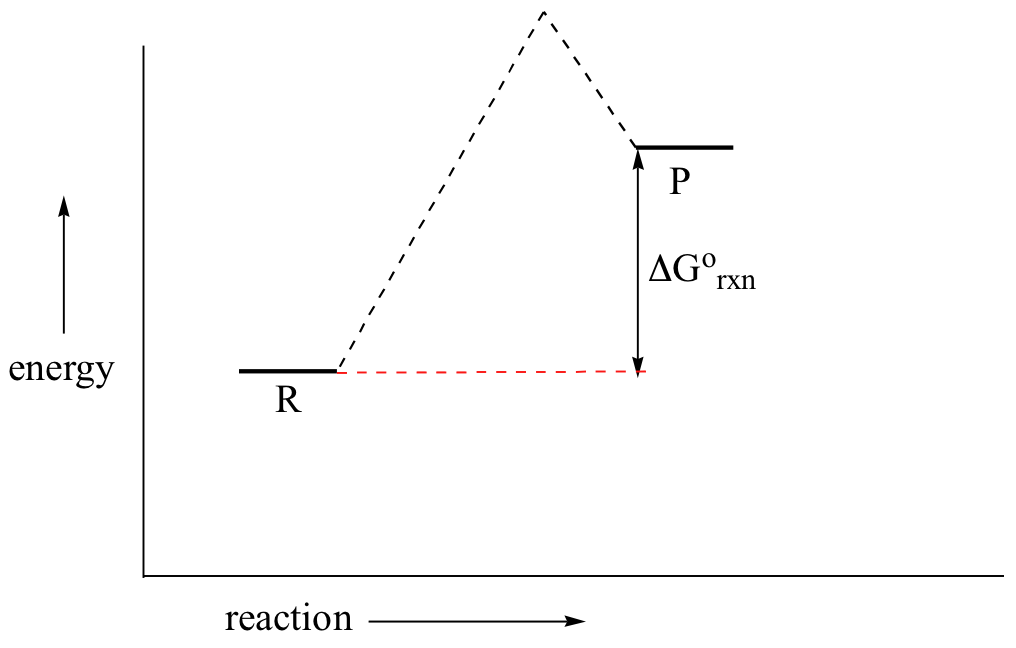 6 2 Energy Diagrams Chemistry Libretexts
6 2 Energy Diagrams Chemistry Libretexts
Illustrated Glossary Of Organic Chemistry Rate Determing Step
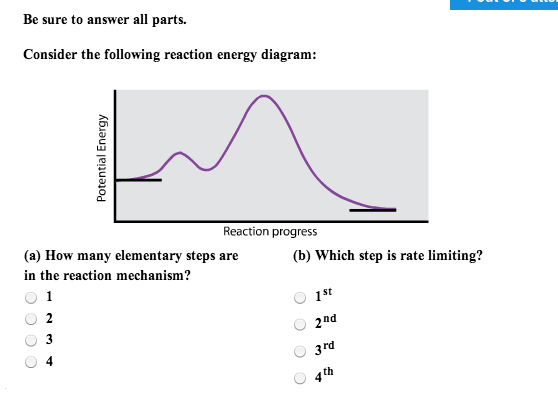 Solved Consider The Following Reaction Energy Diagram Ho
Solved Consider The Following Reaction Energy Diagram Ho
 Quiz 8 1 Bromobutane Question 1 Which One Of The Energy Diagrams
Quiz 8 1 Bromobutane Question 1 Which One Of The Energy Diagrams
Catalysts In 21st Century Energy
How Can I Draw An Elementary Reaction In A Potential Energy Diagram
 Reaction Energy Diagram For The Isomerization Of Glucos Open I
Reaction Energy Diagram For The Isomerization Of Glucos Open I
Organic Chemistry I New Reaction Energy Diagrams
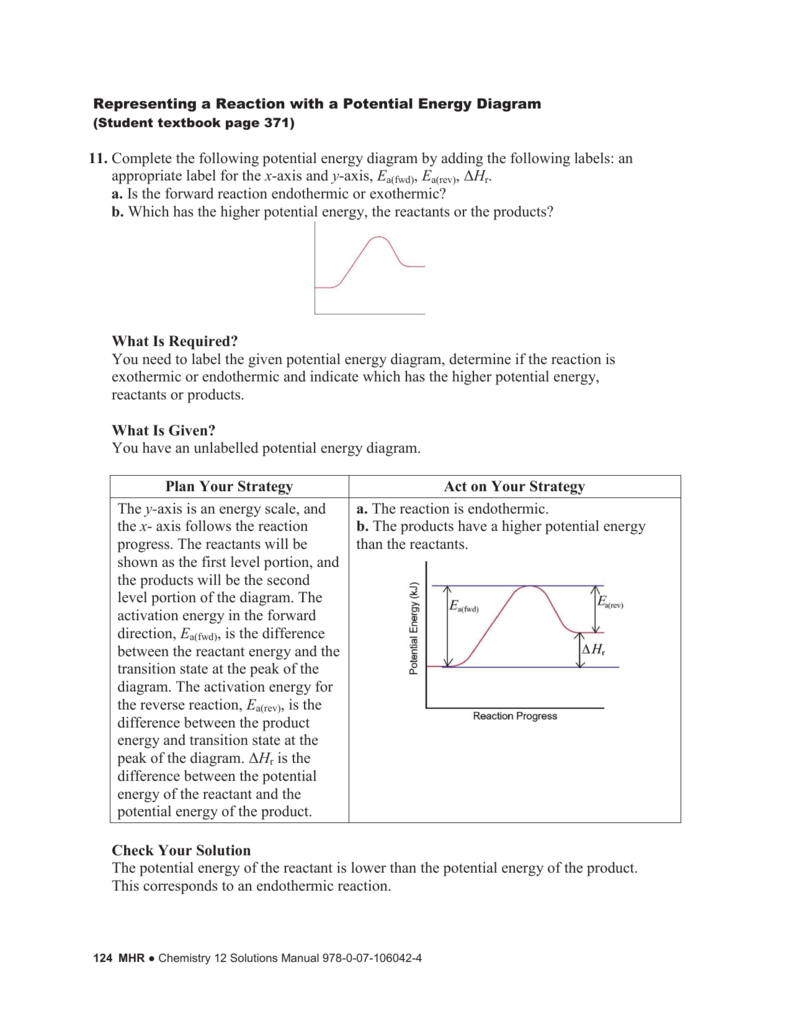 Representing A Reaction With A Potential Energy Diagram
Representing A Reaction With A Potential Energy Diagram
Developer Adoption As A Chemical Reaction Activation Energy And
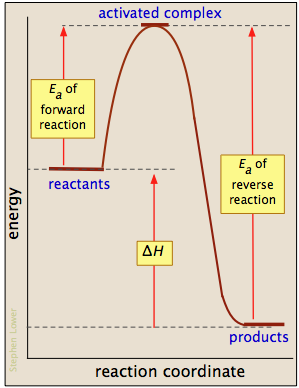 Basics Of Reaction Profiles Chemistry Libretexts
Basics Of Reaction Profiles Chemistry Libretexts
 How To Draw The Curves In An Energy Diagram In R Stack Overflow
How To Draw The Curves In An Energy Diagram In R Stack Overflow
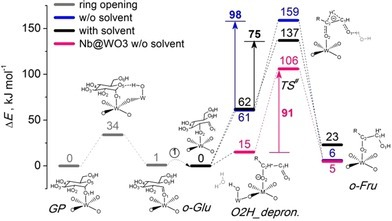
 Reaction Energy Diagram Of O Glu Isomerization To O Fru Over The
Reaction Energy Diagram Of O Glu Isomerization To O Fru Over The
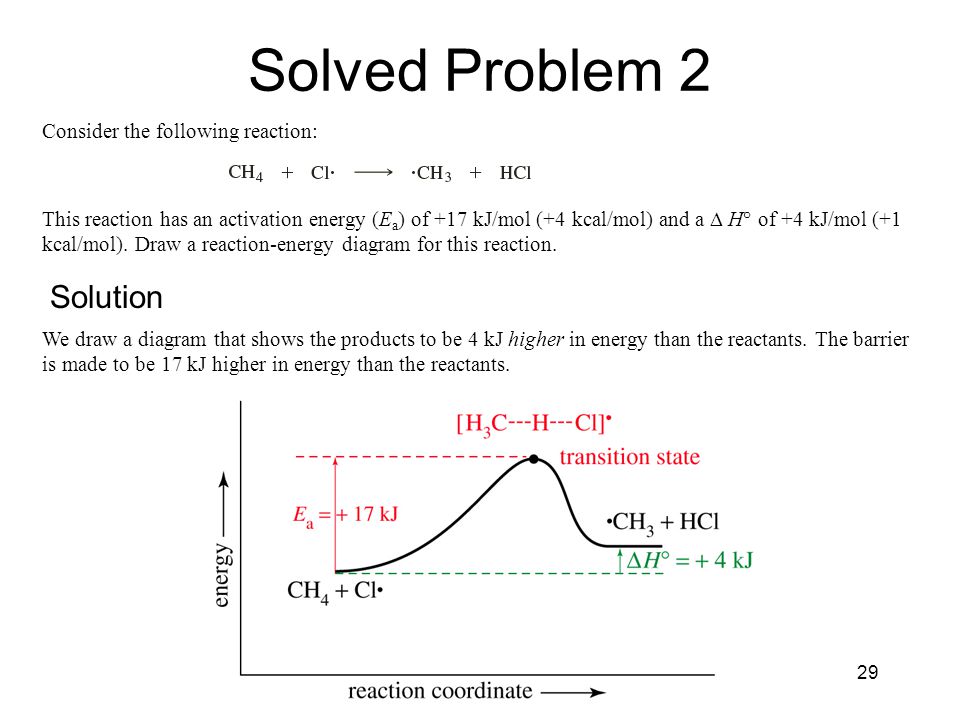 The Study Of Chemical Reactions Ppt Video Online Download
The Study Of Chemical Reactions Ppt Video Online Download
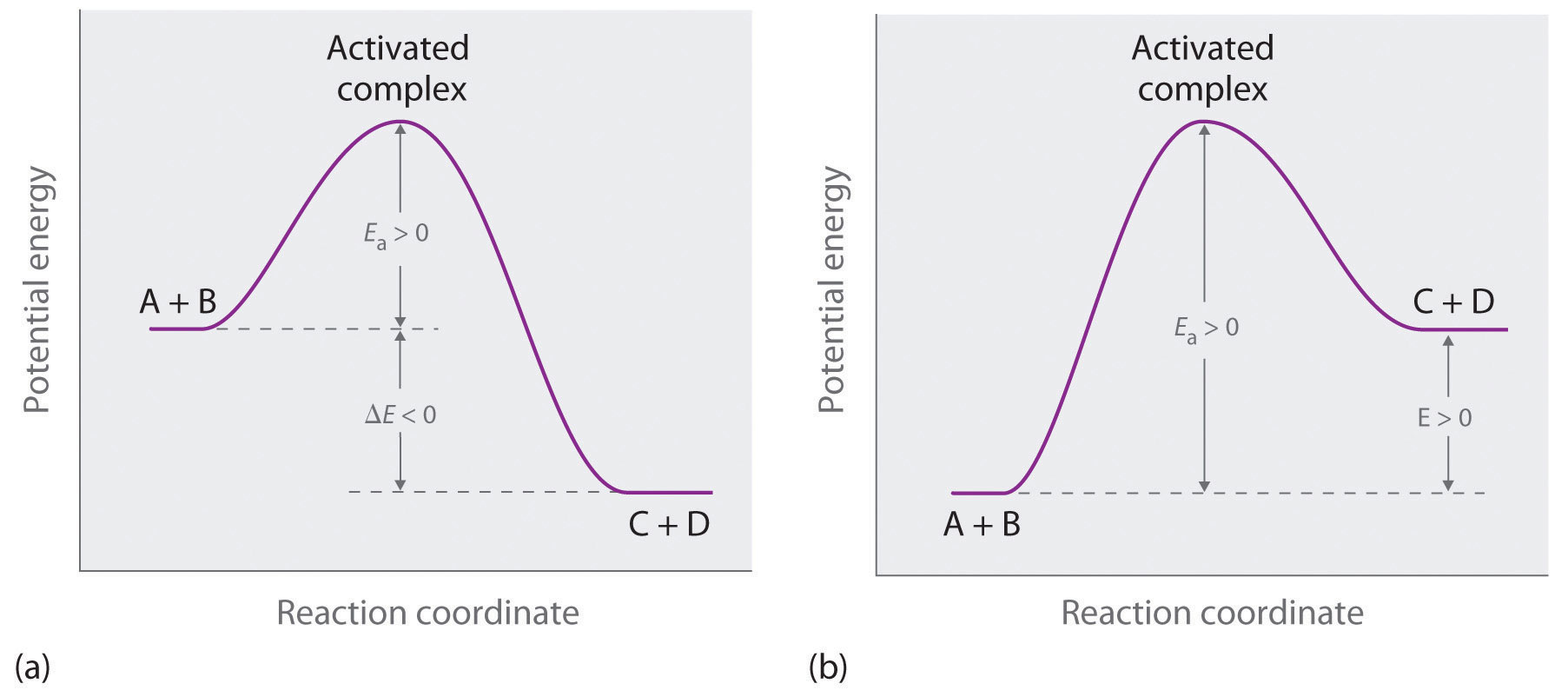
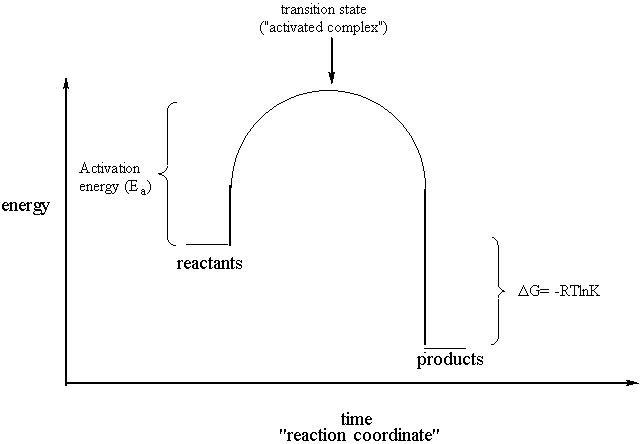
0 Response to "Consider The Following Reaction Energy Diagram"
Post a Comment