Label The Diagram According To The Components And Processes Of A Voltaic Cell
Allows for electron transfer external current. Anode the electrode at which oxidation occurs.
 Electrochemistry Potentiometry
Electrochemistry Potentiometry
The half cell is a chamber in the voltaic cell where one half cell is the site of the oxidation reaction and the other half cell is the site of the reduction reaction.
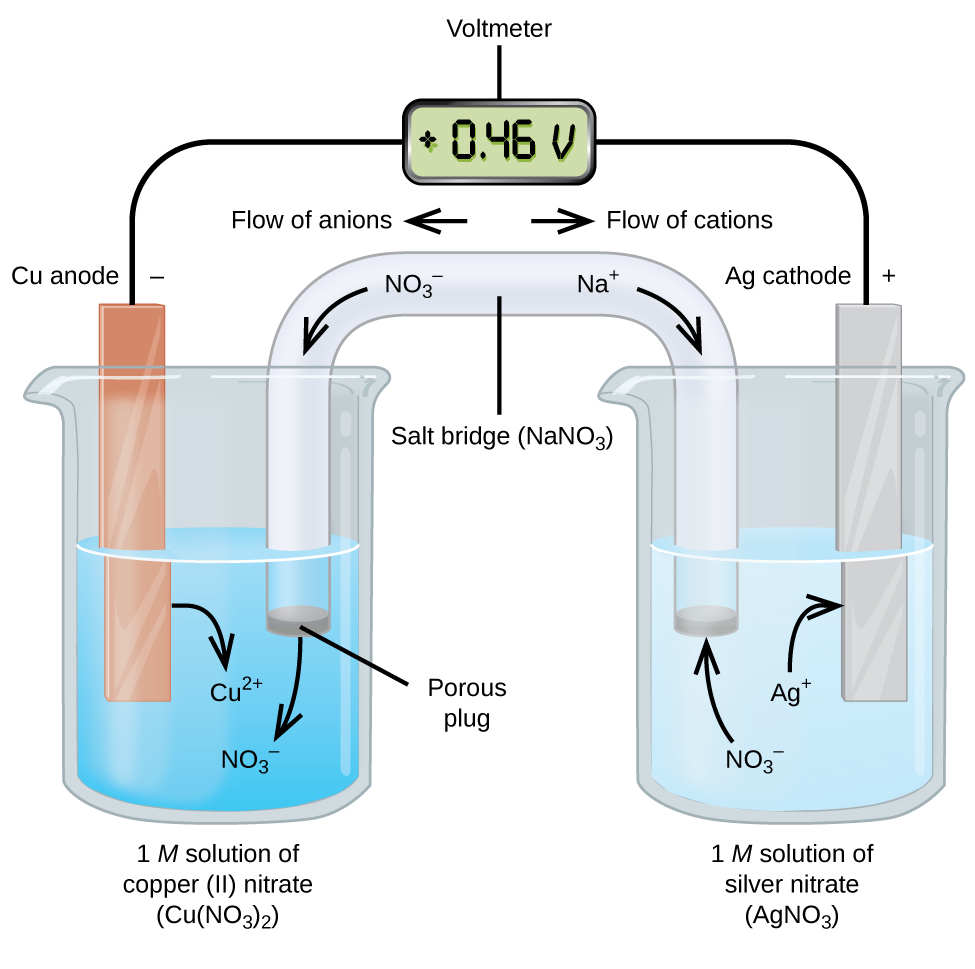
Label the diagram according to the components and processes of a voltaic cell. Thus the salt bridge will help the migration of ions across the two compartments or two half cells. The voltaic cell requires a reducing agent an oxidizing agent a salt bridge a cathode an anode and a wire connecting the cathode and anode. Label the diagram according to the components and processes.
The electrode at which reduction occurs electrons flow into the cathode electrodes. Label the anode and the cathode and indica. A voltaic cell consists of two half cells.
482 6 and practice problems 3 4 6 coo 103. The other is a copper metal strip in a solution of a copper salt. The electrode at which oxidation occurs electrons leave the anode cathode.
Label the anode and cathode. Make a sketch of a concentration cell employing two znzn2 half cells. These are called a zinc electrode the and copper electrode the respectively.
Drag the appropriate labels to their respective targets. Consider the electrolytic cell. Show transcribed image text label the diagram according to the components and processes of a voltaic cell.
Draw an electrolytic cell in which mn2 is reduced to mn and sn is oxidized to. Draw a diagram of the cell and label the electrodes electrolytes direction of electron flow and direction if ion movement from the salt bridge. Consider the concentration cell.
A voltaic cell has several components o o o o o o anode. If a copper zinc voltaic cell utilizes znso4 and cuso4 solution you will use a saturated na2so4 solution in the salt bridge. Label the diagram according to the components and processes of a voltaic cell.
Label the diagram according to the components and processes of a voltaic cell. Cathode the electrode at which reduction occurs. H 20 ccs i cclq now try pg.
Usually metal conductors that carry electrons in and out of the cell ie. Label the diagram according to the components and processes of a voltaic cell. If the cell is made by using separate half cell containers then some kind of salt bridge must be used to connect the half cells.
Here one is a zinczinc ion half cell where a strip of zinc metal is in a solution of a zinc salt. Label the diagram according to the components and processes of a voltaic cell. The voltaic cell is a method to separate these two reactions and create these currents from moving electrons.
Electrolytes containing the ions involved in the redox reaction. The half cell is a chamber in the voltaic cell where one half cell is the site of the oxidation reaction and the other half cell is the site of the reduction reaction. Write the half reactions and the overall redox reaction that occur in the voltaic cell.
 A Student Is Given A Standard Galvanic Cell Represented Above That
A Student Is Given A Standard Galvanic Cell Represented Above That
 Experiments In Electrochemistry
Experiments In Electrochemistry
 Daniell Cell Study Material For Iit Jee Askiitians
Daniell Cell Study Material For Iit Jee Askiitians
 How Battery Is Made Material Production Process Manufacture
How Battery Is Made Material Production Process Manufacture
 Galvanic And Electrolytic Cells Electrochemical Reactions Siyavula
Galvanic And Electrolytic Cells Electrochemical Reactions Siyavula
 How Does A Galvanic Cell Work Science Abc
How Does A Galvanic Cell Work Science Abc
 20 3 Voltaic Cells Chemistry Libretexts
20 3 Voltaic Cells Chemistry Libretexts
 Sodium Pentachlorophenate C6cl5ona Pubchem
Sodium Pentachlorophenate C6cl5ona Pubchem
 Applications Of Redox Reactions Voltaic Cells Introductory
Applications Of Redox Reactions Voltaic Cells Introductory
 Electrodes And Voltage Of Galvanic Cell Video Khan Academy
Electrodes And Voltage Of Galvanic Cell Video Khan Academy
 Galvanic And Electrolytic Cells Electrochemical Reactions Siyavula
Galvanic And Electrolytic Cells Electrochemical Reactions Siyavula
 Battery And Cell Chemistries Battery Primer
Battery And Cell Chemistries Battery Primer
 Electrochemical Cell Wikipedia
Electrochemical Cell Wikipedia

0 Response to "Label The Diagram According To The Components And Processes Of A Voltaic Cell"
Post a Comment