How To Fill Out Molecular Orbital Diagram
How to fill out a molecular orbital energy level diagram post by junghyukpark1i thu nov 03 2016 430 am i know that this might be hard to put into words but what are the general rules on filling out a molecular orbital energy level diagram ie. Two atomic orbitals in phase create a larger electron density which leads to the σ orbital.
In phase and out of phase wave combinations.
How to fill out molecular orbital diagram. Atomic valence electrons shown in boxes on the left and right fill the lower energy molecular orbitals before the higher ones. We put two electrons into each of the σ1s σ1s σ2s σ2s and σ2p. You can see that the antibonding orbitals are higher in energy and bonding orbitals are lower in energy.
This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion h2. Dont worry this is easier than it seems. The superposition of the two 1s atomic orbitals leads to the formation of the σ and σ molecular orbitals.
Inside the dashed lines are the possible molecular orbitals they are capable of forming. Energy is on the y axis. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1 s electrons on both oxygen atoms and concentrate on the interactions between the 2 s and 2 p valence orbitals.
Each o atom contains 8 electrons so we have 16 electrons to put into the molecular orbitals of o₂. You use the same rules to fill molecular orbitals. Orbital filling diagrams essentially just turn this big list of electron locations into a picture that shows not just what type of electrons an orbital resides in but also which of those orbitals theyre located in.
How to fill in electrons into bondingantibonding orbitals how to determine which is bonding. Lets apply this to the molecular orbital diagram of oxygen. Theory we will formalize a definition of bond order the number of bonds between atoms in a molecule.
The regions to the left and right side of the dashed lines are atomic orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined. To further demonstrate the consistency of the lewis structures with mo.
If the two 1s orbitals are not in phase a node between them causes a jump in energy the σ orbital. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital.
 Electronic Spectroscopy Interpretation Chemistry Libretexts Draw
Electronic Spectroscopy Interpretation Chemistry Libretexts Draw
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Delocalized Bonding And Molecular Orbitals
Delocalized Bonding And Molecular Orbitals
 Molecular Nitrogen And Related Diatomic Molecules
Molecular Nitrogen And Related Diatomic Molecules
 Molecular Orbital Theory Mot Chemistry Study Material
Molecular Orbital Theory Mot Chemistry Study Material
 Organic Chemistry Draw A Simplified Mo Diagram For The Pi System
Organic Chemistry Draw A Simplified Mo Diagram For The Pi System
 Solution Utilize The Molecular Orbital D Chemistry
Solution Utilize The Molecular Orbital D Chemistry
 Molecular Orbital Mo Diagram Of H2 Youtube
Molecular Orbital Mo Diagram Of H2 Youtube
 11 5 Molecular Orbital Theory Chemistry Libretexts
11 5 Molecular Orbital Theory Chemistry Libretexts
 Molecular Orbital Diagram For Oxygen The Occupation Of The
Molecular Orbital Diagram For Oxygen The Occupation Of The
 Introduction To Molecular Orbital Theory
Introduction To Molecular Orbital Theory
 Molecular Orbital Mo Diagram Of O2 Youtube
Molecular Orbital Mo Diagram Of O2 Youtube
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Molecular Orbitals Introductory Chemistry 1st Canadian Edition
Molecular Orbitals Introductory Chemistry 1st Canadian Edition
 Molecular Orbital Diagram For A Simple Pi Bond Bonding And
Molecular Orbital Diagram For A Simple Pi Bond Bonding And
 Molecular Orbital Diagrams Simplified Megan Lim Medium
Molecular Orbital Diagrams Simplified Megan Lim Medium
 Molecular Orbital Diagram Wikipedia
Molecular Orbital Diagram Wikipedia
 Sparknotes Molecular Orbitals Molecular Orbital Theory
Sparknotes Molecular Orbitals Molecular Orbital Theory
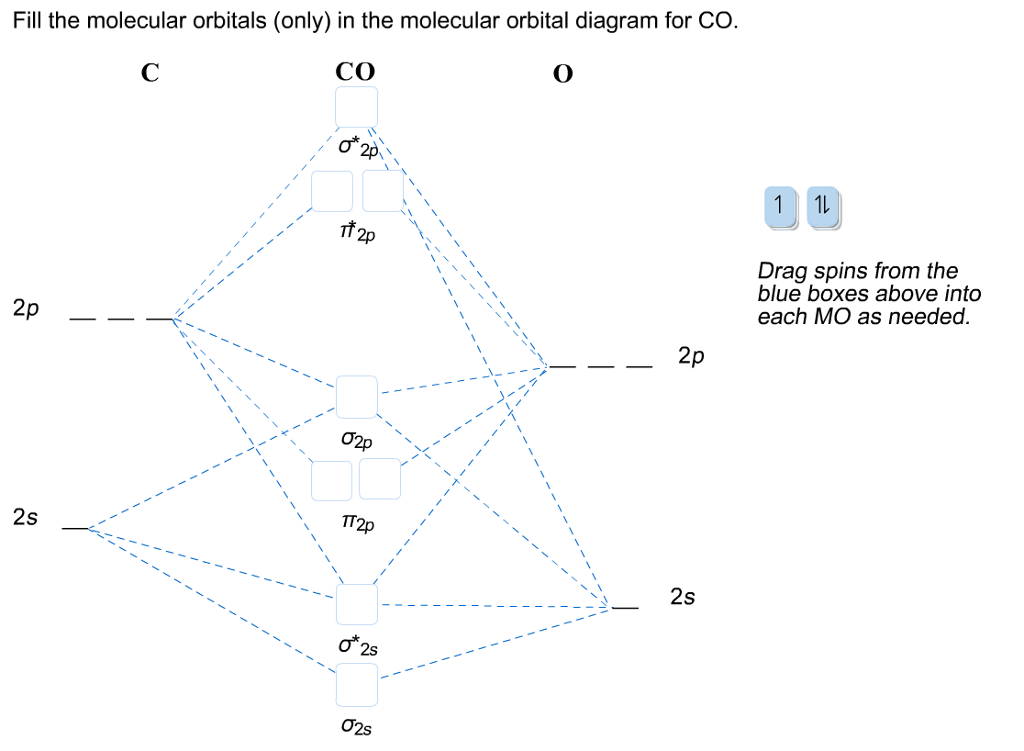
 Solved Fill The Molecular Orbitals Only In The Molecula
Solved Fill The Molecular Orbitals Only In The Molecula
 Molecular Orbitals In Fluorine
Molecular Orbitals In Fluorine



0 Response to "How To Fill Out Molecular Orbital Diagram"
Post a Comment