Use The Mo Diagram Provided Below To Answer The Following Questions
Use the diagram below to answer the following questions. Information from the mo diagram justify o2s stability and show that its bonding order is 2.
 Mo Diagram For N2 Molecular Orbital Youtube
Mo Diagram For N2 Molecular Orbital Youtube
Write formulas for compounds formed from the following elements and provide a compound name.
Use the mo diagram provided below to answer the following questions. Number of bonding valence electrons number of antibonding valence electrons c2 bond order this corresponds to. 7 2 2 52 25. This is paramagnetic because there is 1 unpaired electron sigma 2p1 is missing one.
The relative energies of the sigma orbitals drop below that of the pi orbitals. Potassium and oxygen c. B for n2 the mo diagram is.
The following four spheres represent an mg atom an mg2 ion a s atom and a s2 ion not necessarily in that order. In o 2 and f 2 there is a crossover of the sigma and the pi ortbials. Aluminum and sulfur d.
Sigma 2s2 sigma 2s2 pi 2p4 sigma 2p1. Single bond double bond triple bond half of a bond between a single and double bond between a double and a triple bond no bond c2 does not form. Use the diagram below to answer the following.
The molecular orbital energy level diagram provided shows the energies of the orbitals for the valence electrons in the c 2 molecule. Use the molecular orbital energy diagram below to answer the questions about bond order for the m. Molecular orbitals mo are constructed from atomic orbitals.
Sodium and bromine b. So the bond order is 8 2 2 62 3. Barium and chlorine e.
The value of x is the measure of 1 is the measure of 2 is the measure of 3 is the measure of 4 is. For the species n2 is diamagnetic because it has no unpaired electrons. Use the diagram below to answer the following questions.
Silver i and chlorine 7. Lithium and oxygen f. 155 please select the best answer from the choices provided.
Ln bisects klm into two congruent angles measuring 3x 4 and 4x 27 find mklm. Use this mo diagram to answer the questions below according to molecular orbital theory which of the following species is the most likely to exist ie which will have the greatest bond order. The following diagram shows the molecular orbital energy level diagrams for the.
Use your knowledge about the relative sizes of atoms cations and anions to determine which of the following sets of reactions is most consistent with the sizes of the atoms and ions shown below. Analyze the diagram below and complete the instructions that follow. Chem1101 2014 j 5 june 2014.
 Solved The Molecular Orbital Diagram Of N 2 From Your Tex
Solved The Molecular Orbital Diagram Of N 2 From Your Tex
 Diatomic Species Mo Theory Chemogenesis
Diatomic Species Mo Theory Chemogenesis
 What Is The Molecular Orbital Diagram For O2 And O2 Ions Quora
What Is The Molecular Orbital Diagram For O2 And O2 Ions Quora
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Solved Using The Cooling Curves Provided Below Construct
Solved Using The Cooling Curves Provided Below Construct
 8 4 Molecular Orbital Theory Chemistry
8 4 Molecular Orbital Theory Chemistry
 Diagrams Charts And Graphs View As Single Page
Diagrams Charts And Graphs View As Single Page
 What Is An F2 Bond Order Quora
What Is An F2 Bond Order Quora
 Contrasting Mo And Vb Theory Chemistry Libretexts
Contrasting Mo And Vb Theory Chemistry Libretexts
 10 5 Molecular Orbital Theory Chemistry Libretexts
10 5 Molecular Orbital Theory Chemistry Libretexts
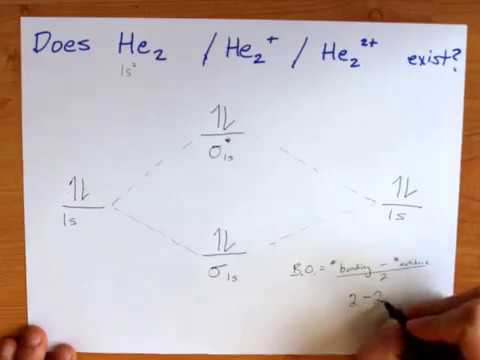
 Do He2 He2 He2 2 Exist Stable Molecular Orbital Theory
Do He2 He2 He2 2 Exist Stable Molecular Orbital Theory
 Mechanical Engineering Recent Questions Chegg Com
Mechanical Engineering Recent Questions Chegg Com
 Solved 1 Use The Molecular Orbital Diagram Below To Answ
Solved 1 Use The Molecular Orbital Diagram Below To Answ
 Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
Chemical Bonding Molecular Orbitals Of H2 And He2 Britannica Com
 Mo Diagrams For Diatomic Molecules
Mo Diagrams For Diatomic Molecules
 Definition Of Shear And Moment Diagrams Chegg Com
Definition Of Shear And Moment Diagrams Chegg Com
 Introduction To Inorganic Chemistry Molecular Orbital Theory
Introduction To Inorganic Chemistry Molecular Orbital Theory
 10 5 Molecular Orbital Theory Chemistry Libretexts
10 5 Molecular Orbital Theory Chemistry Libretexts


0 Response to "Use The Mo Diagram Provided Below To Answer The Following Questions"
Post a Comment