What Is The Activation Energy For The Reaction In This Energy Diagram
The answer is 125 kjmol 216 kjmol 341 kjmol. At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products.
 What Is The Activation Energy For The Reverse Reaction In Terms Of
What Is The Activation Energy For The Reverse Reaction In Terms Of
The activation energy of the reverse reaction should be equal to the activation energy of the forward reaction minus the drive in the reaction delta e.
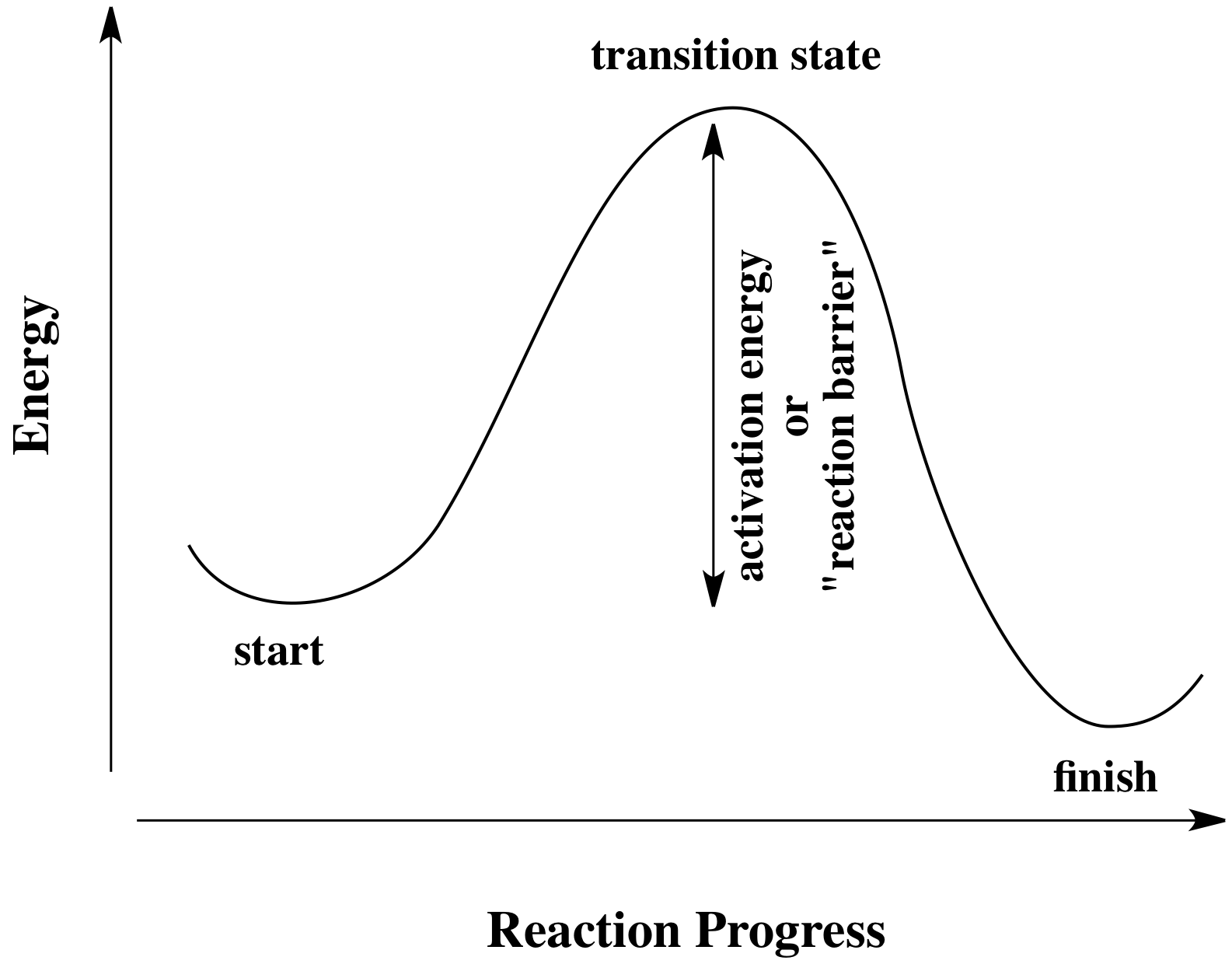
What is the activation energy for the reaction in this energy diagram. If the reaction were to proceed in the reverse direction endergonic the transition state would remain the same but the activation energy would be larger. The activation energy for the following reaction is 125 kjmol. In chemistry and physics activation energy is the energy which must be available to a chemical or nuclear system with potential reactants to result in.
In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Select all that apply a catalyst lowers the activation energy. In energy profile diagram it is the difference in energy from the reactants to topmost peak of the graph.
This state is also known as an activated complex. Activation energy of a reaction is the energy required by which the molecules must collide to give a successful product. At the peak of the activation energy hump the reactants are in the transition state halfway between being reactants and forming products.
The horizontal axis represents the the sequence of infinitesimally small changes that must occur to convert the reactants into the products of this reaction. The activation energy shown in the diagram below is for the forward reaction reactants products which is exergonic. This state is also known as an activated complex.
Activation energy is the difference between the starting amount of energy of the reactants and the energy of the activated complex. The vertical axis in this diagram represents the free energy of a pair of molecules as a chlorine atom is transferred from one to the other. The activation energy ea of a reaction is measured in joules.
A chemical reaction nuclear reaction or various other physical phenomena. The energy of the reactants of the reverse reaction is 13 kj the activated complex is still at 83 kj. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled.
This site might help you.
 Gcse Chemistry What Are Energy Level Diagrams What Is The
Gcse Chemistry What Are Energy Level Diagrams What Is The
 Reaction Energy Profiles Activation Energy Exothermic Endothermic
Reaction Energy Profiles Activation Energy Exothermic Endothermic
 Potential Energy Diagram With Without Catalyst In A Hypothetical
Potential Energy Diagram With Without Catalyst In A Hypothetical
 Reaction Coordinate Diagrams College Chemistry
Reaction Coordinate Diagrams College Chemistry
 Activation Energy And Temperature Dependence Boundless Chemistry
Activation Energy And Temperature Dependence Boundless Chemistry
 The Activation Energy Of Chemical Reactions
The Activation Energy Of Chemical Reactions
 Reaction Energy Diagram Sn1 Youtube
Reaction Energy Diagram Sn1 Youtube
 Energy Diagram Module Series Part Three Intermediates And Rate
Energy Diagram Module Series Part Three Intermediates And Rate
 Potential Energy Diagram Problem Set Part 1
Potential Energy Diagram Problem Set Part 1
 Activation Energy Of Enzymes Definition Calculation Example
Activation Energy Of Enzymes Definition Calculation Example
 Endothermic Reaction Energy Diagram New Activation Energy Wiring
Endothermic Reaction Energy Diagram New Activation Energy Wiring
 Kinetics Reaction Rates Collision Theory Factors Affecting Reaction
Kinetics Reaction Rates Collision Theory Factors Affecting Reaction
 Solved The Graph Represents The Energy Diagram For A Chem
Solved The Graph Represents The Energy Diagram For A Chem
 Answer Label The Energy Diagram For A Two Clutch Prep
Answer Label The Energy Diagram For A Two Clutch Prep
 Organic Chemistry I New Reaction Energy Diagrams
Organic Chemistry I New Reaction Energy Diagrams
 How To Read Potential Energy Diagrams
How To Read Potential Energy Diagrams

0 Response to "What Is The Activation Energy For The Reaction In This Energy Diagram"
Post a Comment