What Is A Lone Pair In A Lewis Diagram
A single shared pair of electrons is called a single bond. The lewis diagram for nh3 is.
 Draw The Lewis Structure For Butanal Which Has The Condensed
Draw The Lewis Structure For Butanal Which Has The Condensed
There are lone pairs around the central atom so the geometry of pooh3 is.

What is a lone pair in a lewis diagram. They are also referred to in the chemistry of lewis acids and bases. When one or more of the bonding pairs of electrons is replaced with a lone pair the molecular geometry actual shape of the molecule is altered. However not all non bonding pairs of electrons are considered by chemists to be lone pairs.
A lewis structure is a graphic representation of the electron distribution around atoms. A dash or line is sometimes used to indicate a shared pair of electrons. In other words a lone pair is a non bonding pair.
The lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms. The lewis diagram for seh2 is. Jump to navigation jump to search.
Lone pair is a concept used in valence shell electron pair repulsion theory vsepr theory which explains the shapes of molecules. And because the non bonding nitrogen lone pair lies fairly close to nitrogen it compresses the h n h bond down from 1095 at to approx. Lewis structures also known as lewis dot diagrams lewis dot formulas lewis dot structures electron dot structures or lewis electron dot structures leds are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
The electron pair geometry around the se atom in seh2 is. The total number of electron pairs both bonding pairs and lone pairs leads to what is called the electron domain geometry. On other hand the lone pair explains the basicity of the ammonia molecule.
On the other hand a bonding pair is 2 electrons from different atoms which overlap to form a pi or sigma bond. A lone pair consists of 2 electrons in the same orbital from the same atom which are not involved in bonding. Ammonium ion nh4 is a regular tetrahedron.
The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. There are lone pairs around the central atom so the geometry of seh2 is. The electron pair geometry around the n atom in nh3 is tetrahedral.
Sparknotes Organic Chemistry Covalent Bonding Covalent Bonds And
 Draw The Lewis Structure For Hcn Include Lone Pairs
Draw The Lewis Structure For Hcn Include Lone Pairs
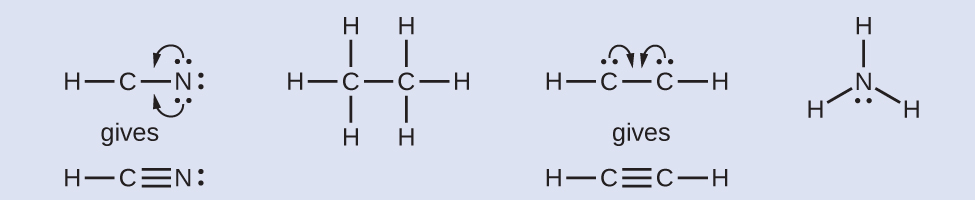 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 Are Lone Pairs Of Electrons Involved In Chemical Bonding Socratic
Are Lone Pairs Of Electrons Involved In Chemical Bonding Socratic
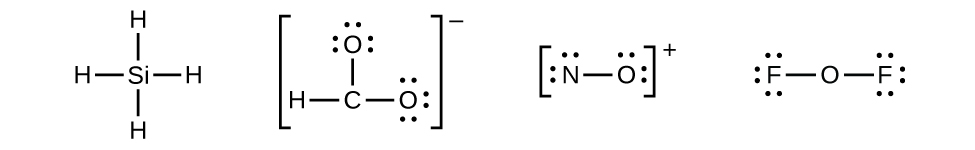 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
 In The Lewis Structure For Formic Acid Hcooh How Ma
In The Lewis Structure For Formic Acid Hcooh How Ma
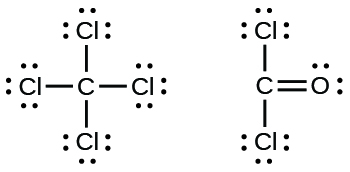 7 3 Lewis Symbols And Structures Chemistry
7 3 Lewis Symbols And Structures Chemistry
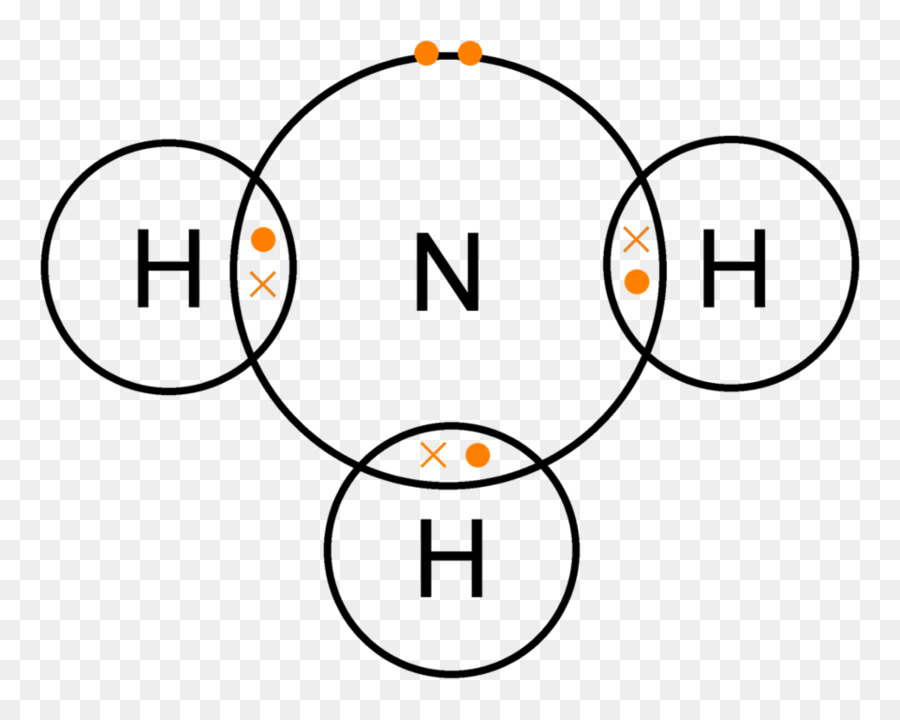 Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond Dot
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond Dot
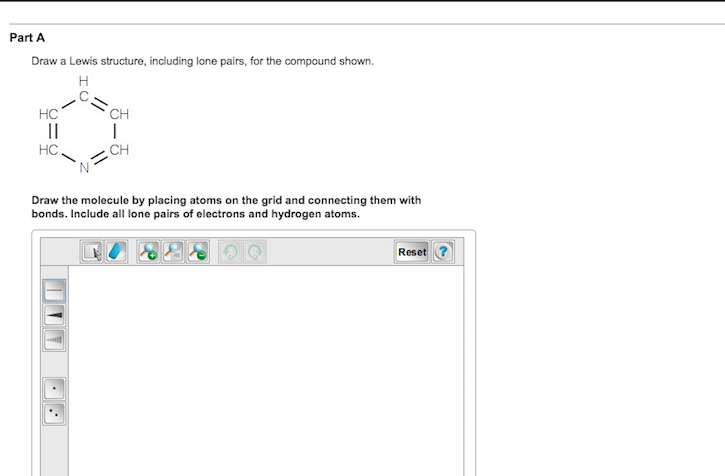 Solved Draw A Lewis Structure Including Lone Pairs For
Solved Draw A Lewis Structure Including Lone Pairs For
1 How Many Lone Pair Around C Atom In Ch4 So3 2 Lewis Structure 9
Lewis Structures And Molecular Shapes
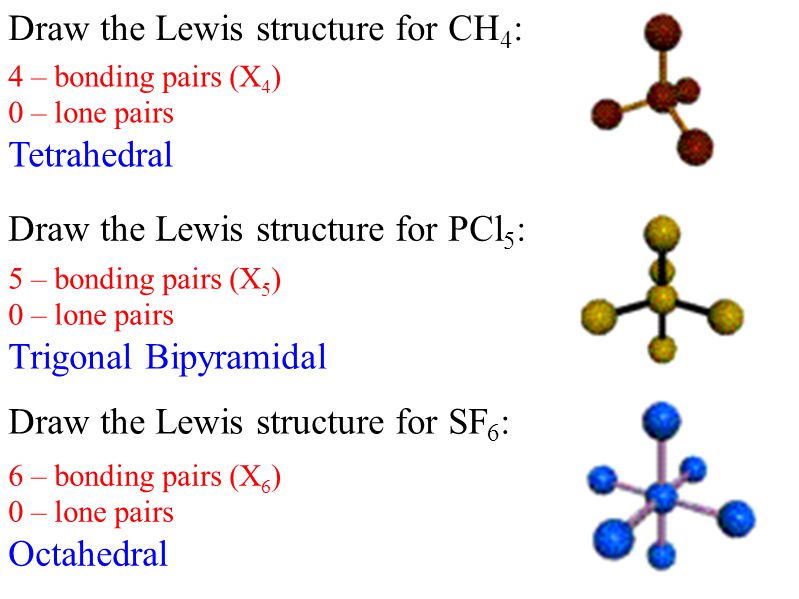 Drawing Lewis Structures And Vsepr Draw Basic Lewis Dot Structures
Drawing Lewis Structures And Vsepr Draw Basic Lewis Dot Structures
 Brf5 Lewis Structure How To Draw The Lewis Dot Structure For Brf5
Brf5 Lewis Structure How To Draw The Lewis Dot Structure For Brf5
 Solved Draw The Lewis Structure Of Xecl 4 Showing All Lon
Solved Draw The Lewis Structure Of Xecl 4 Showing All Lon
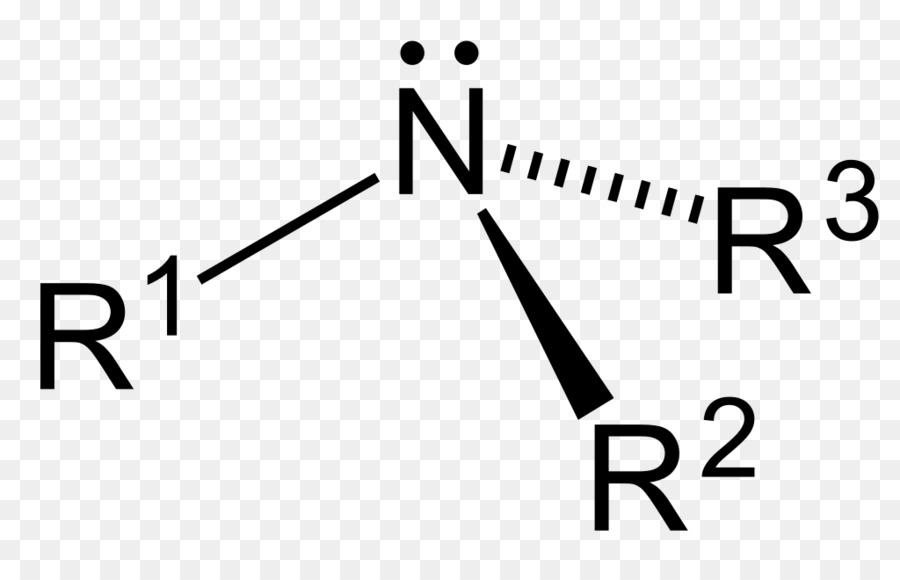 Ammonium Ammonia Lone Pair Ion Lewis Structure General Png
Ammonium Ammonia Lone Pair Ion Lewis Structure General Png
 Solution Add Lone Pairs To These Lewis S Chemistry
Solution Add Lone Pairs To These Lewis S Chemistry
 Unit 5b Covalent Bonding Ppt Video Online Download
Unit 5b Covalent Bonding Ppt Video Online Download
 If Lewis Structure Has Too Few Electrons Add Lone Pairs Of Electrons
If Lewis Structure Has Too Few Electrons Add Lone Pairs Of Electrons
 Resonance Chemistry Libretexts
Resonance Chemistry Libretexts
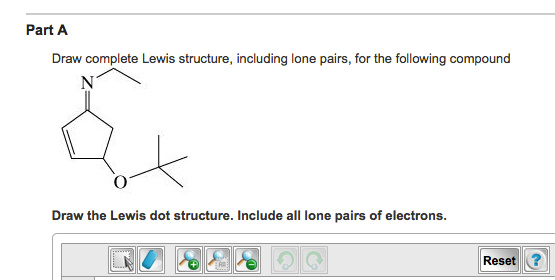 Solved Draw Complete Lewis Structure Including Lone Pair
Solved Draw Complete Lewis Structure Including Lone Pair

0 Response to "What Is A Lone Pair In A Lewis Diagram"
Post a Comment