One Way To Increase The Volume Of The Gas In The Balloon In The Diagram Above Is To
What are 3 completely different ways to increase the volume of gas in a balloon. Will increase volume.
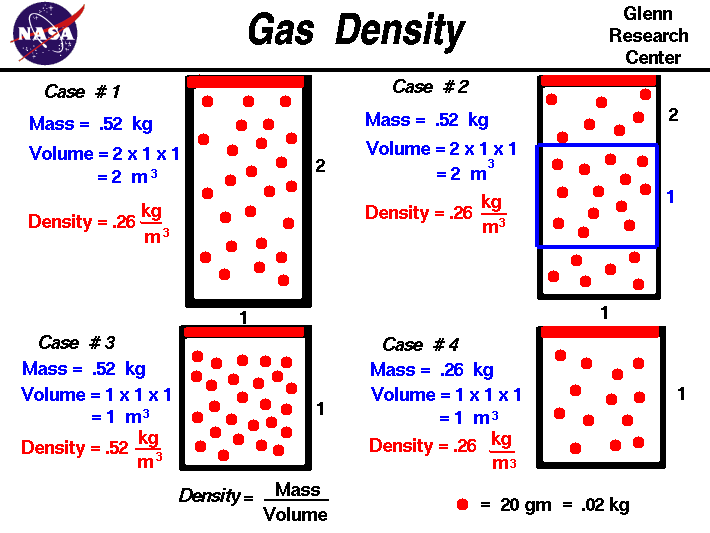
Lecture 6 Ideal Gas Law Rising And Sinking Air
Increase the temperature of the water c.

One way to increase the volume of the gas in the balloon in the diagram above is to. A sample of nitrogen gas is collected. And now for something completely different. When gas is heated in a balloon its volume will increase.
Decreases the pressure by 80 kpa b. Gas in the balloon in the diagram. Place the balloon in a hot environment increase in temp.
If pressure is constant the relationship between temperature and volume is a. What is the new temperature of the gas. A gas has a volume of 500 cm 3 at a temperature of 73ocwhat volume would the gas occupy at a temperature.
Increase the temperature of the water h. Push the balloon farther down into the. The total pressure of an o2 ar he gas mixture is 755 mmhg.
Seal the top of the water bath 6. One way to increase pressure on a gas is to a. Push the balloon farther down into the water bath d.
Increases the pressure by 160 kpa 31. Above is to f. The monatomic gas.
Decreases the pressure by 160 kpa c. If you are melting two candles with one candle being. Seal the top of the water bath.
One way to increase the volume of the. One way to increase the volume of the gas in the balloon in the diagram to the left is to a. Gas laws and intermolecular forces 1.
According to the graph increasing the volume from 100 ml to 150 ml a. Cool the gas in the balloon only. If temperature is constant the relationship between pressure and volume is a.
One way to increase to volume of the gas in the balloon. Temperature so that the balloon shrinks to one quarter of its original volume. An increase in average kinetic energy means that average velocity must be increased ke 5 mv2.
Cool the gas and the balloon only b. Assuming all other conditions stay the same the volume of the balloon will increase as the temperature increases. So an increase in temperature increases the average kinetic energy.
Gas molecules spread out occupy 01 of their volume so far apart that the attractive forces between them are neglible thus all gases are miscible with one another regardless of polarity gases separate based on density not polarity gravity causes more dense gases to settle below light gases explains why hot air rises cause less dense. Kinetic molecular theory of gases is. One of the main assumptions of the.
The molar heat capacity at constant volume of diatomic hydrogen gas h2 is 5r2 at 500 k but only 3r2 at 50 k. Which gas will undergo the greatest increase in temperature. More velocity means that the molecules in the balloon are flying faster and since pressure is the same the molecules spread out more which must increase the volume.
Increases the pressure by 80 kpa d.
 Chemistry 2000 Released Test Items Virginia Department Of Education
Chemistry 2000 Released Test Items Virginia Department Of Education
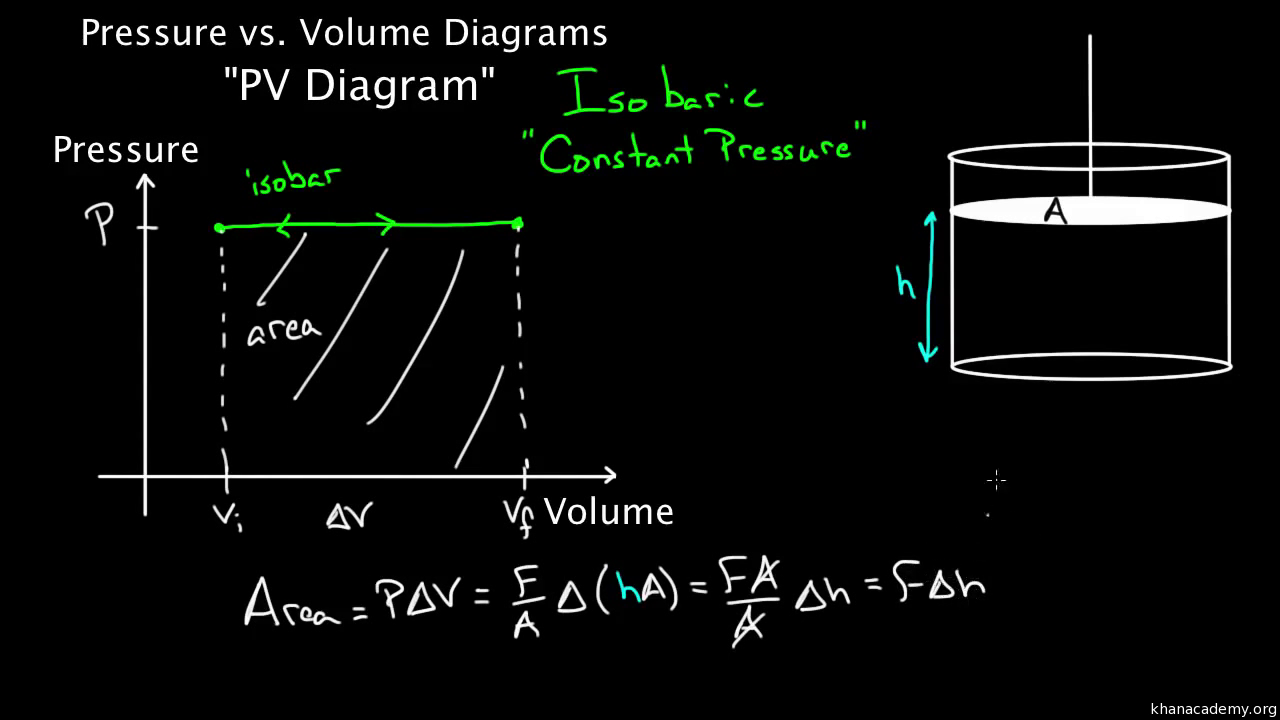 Pv Diagrams Part 1 Work And Isobaric Processes Video Khan Academy
Pv Diagrams Part 1 Work And Isobaric Processes Video Khan Academy
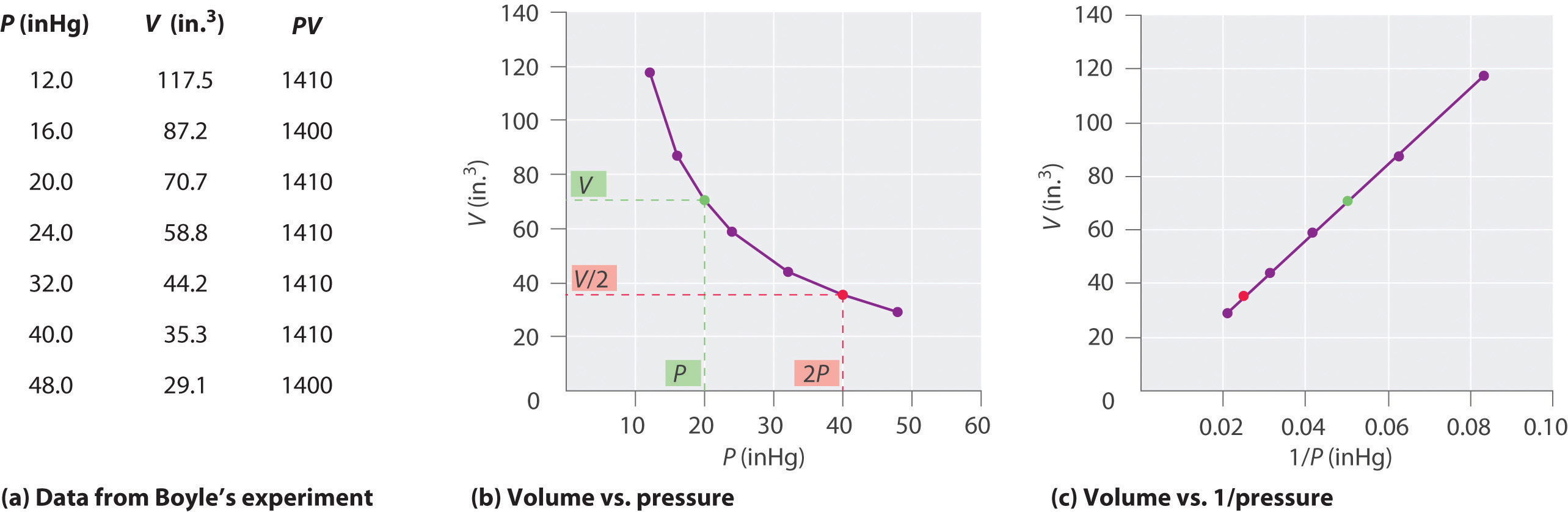 6 3 Relationships Among Pressure Temperature Volume And Amount
6 3 Relationships Among Pressure Temperature Volume And Amount
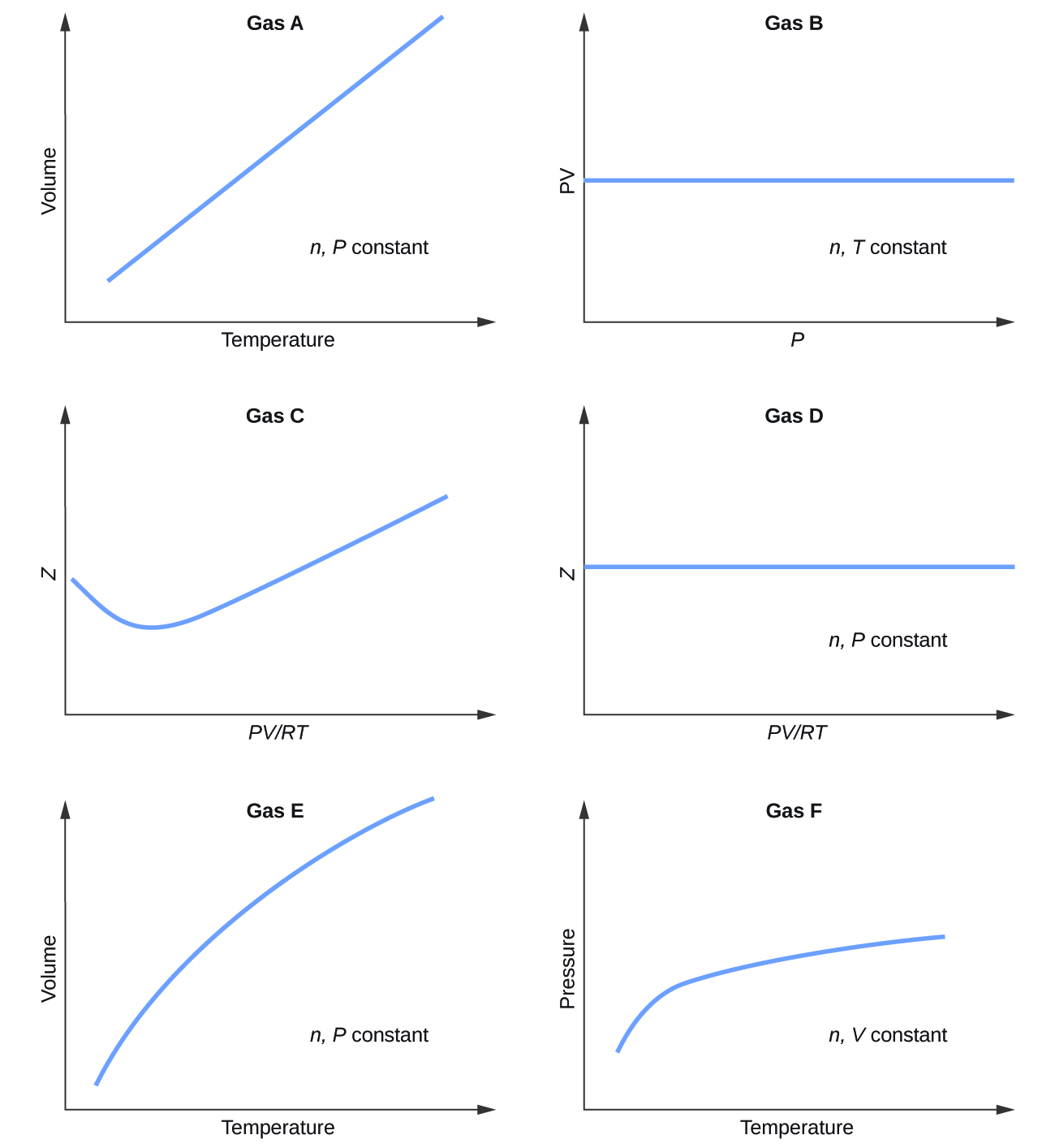 9 E Gases Exercises Chemistry Libretexts
9 E Gases Exercises Chemistry Libretexts
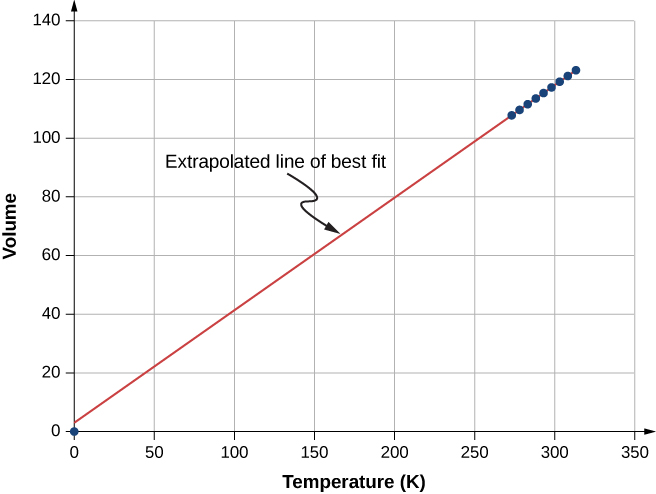 2 1 Molecular Model Of An Ideal Gas Physics Libretexts
2 1 Molecular Model Of An Ideal Gas Physics Libretexts
 3 Ways To Demonstrate Charles S Law Wikihow
3 Ways To Demonstrate Charles S Law Wikihow
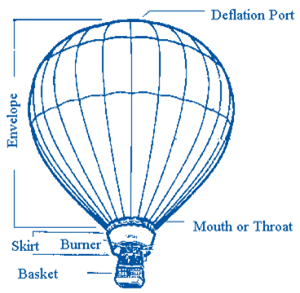
 How Do Hot Air Balloons Work Explain That Stuff
How Do Hot Air Balloons Work Explain That Stuff
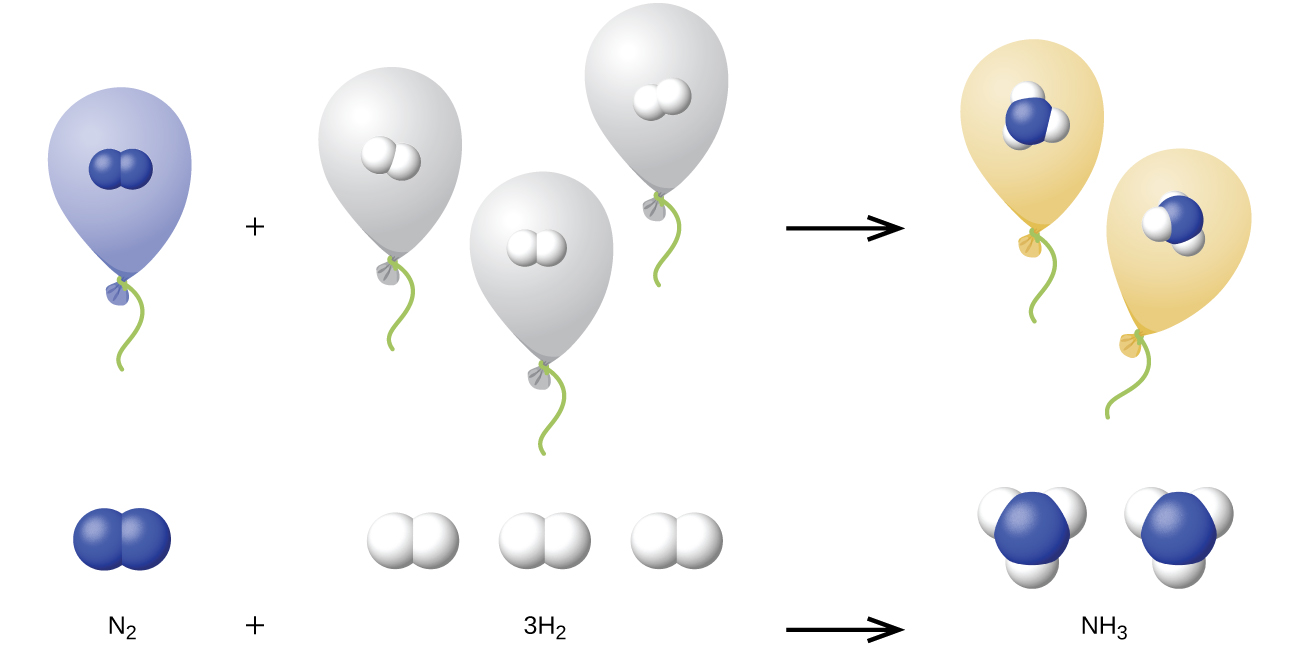 9 3 Stoichiometry Of Gaseous Substances Mixtures And Reactions
9 3 Stoichiometry Of Gaseous Substances Mixtures And Reactions
Lecture 6 Ideal Gas Law Rising And Sinking Air
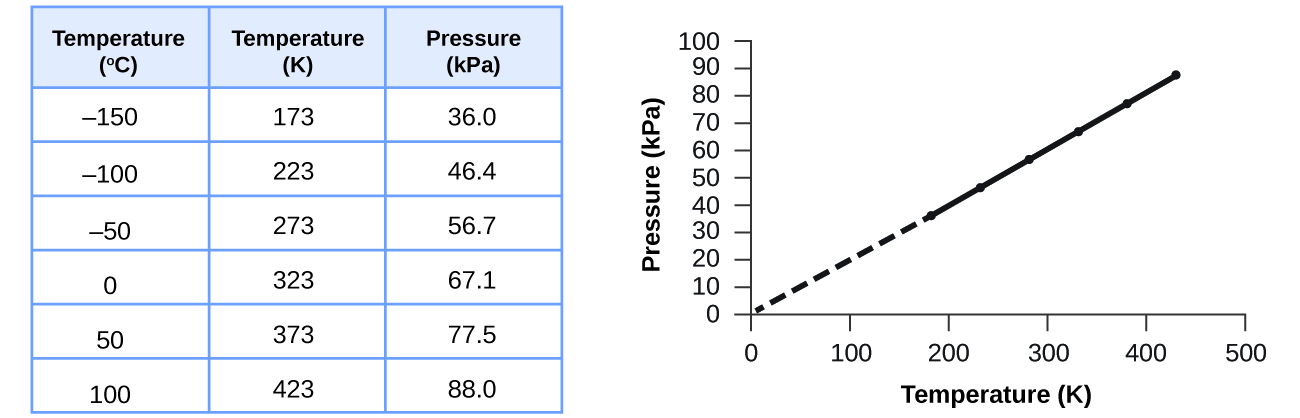 9 2 Relating Pressure Volume Amount And Temperature The Ideal
9 2 Relating Pressure Volume Amount And Temperature The Ideal
 Relating Pressure Volume Amount And Temperature The Ideal Gas
Relating Pressure Volume Amount And Temperature The Ideal Gas
Lecture 6 Ideal Gas Law Rising And Sinking Air
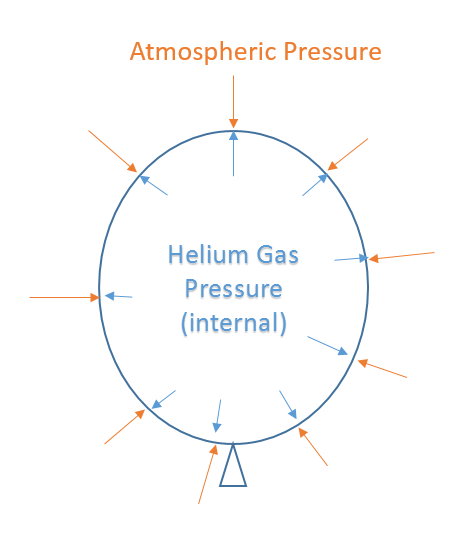
Lecture 6 Ideal Gas Law Rising And Sinking Air
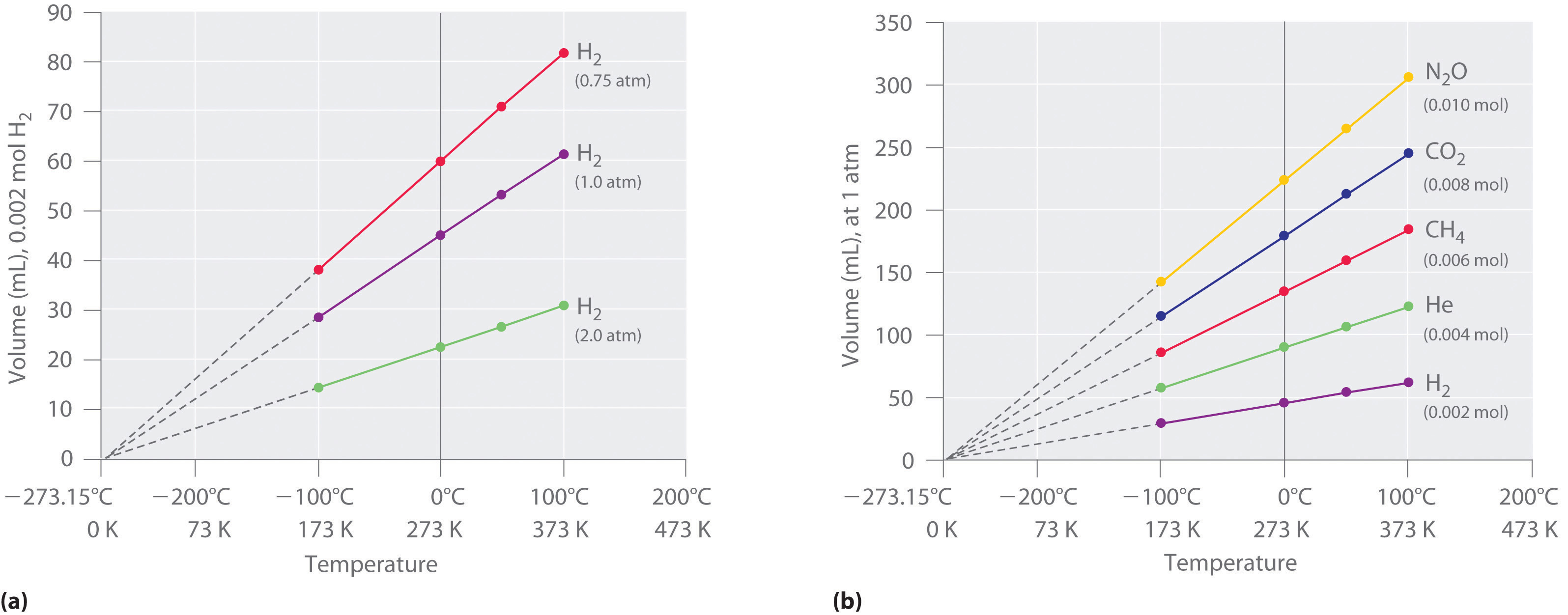 6 3 Relationships Among Pressure Temperature Volume And Amount
6 3 Relationships Among Pressure Temperature Volume And Amount
The Creative Science Centre By Dr Jonathan P Hare

0 Response to "One Way To Increase The Volume Of The Gas In The Balloon In The Diagram Above Is To"
Post a Comment