Electron Distribution Diagram Of Water
Another bond type is the ionic bond. If the electron pairs in covalent bonds were donated and shared absolutely evenly there would be no fixed local.
 The Chemical Context Of Life Ppt Download
The Chemical Context Of Life Ppt Download
These electron sharing diagrams.
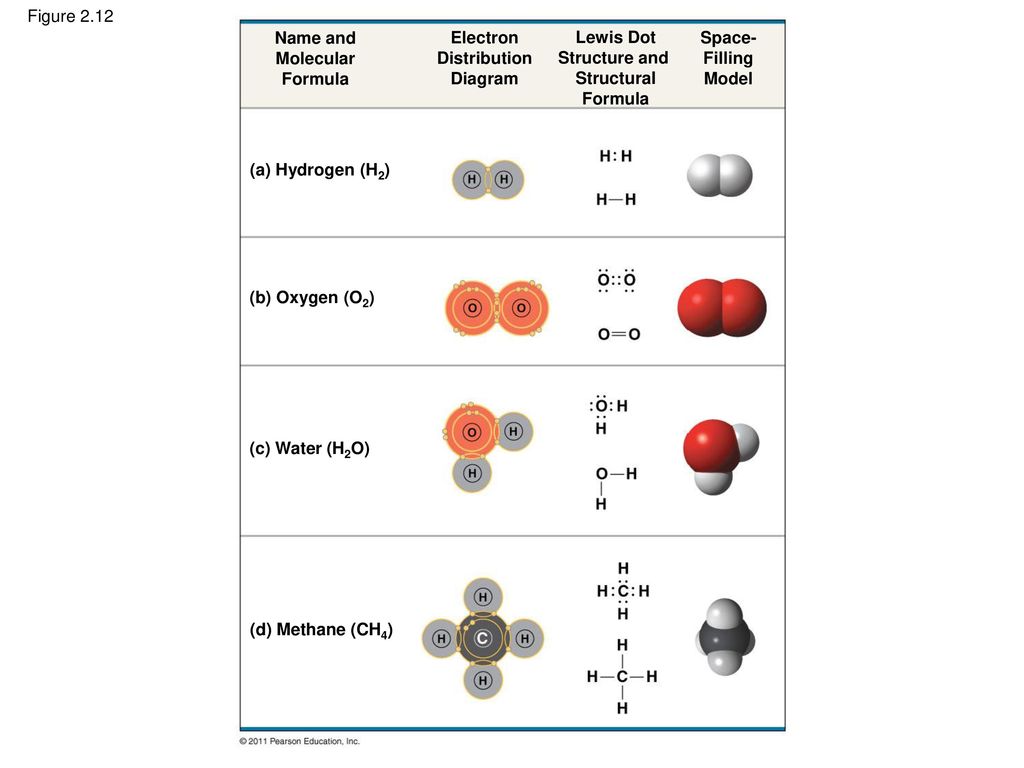
Electron distribution diagram of water. These electron pairs form electron clouds. Indicate the areas with slight negative and positive charges that enable a water molecule to form hydrogen bonds with other polar molecules. Polar covalent bonds occur when one atom is bonded to a more electronegative atom and the electrons of the bond are not shared equally.
For example helium neon and argon are exceptionally stable and unreactive monoatomic gases. The eight outer electrons are often shown as the pairs of dots in where the pairs of electrons between the o and h atoms represent the o h covalent bonds and the other two pairs of electrons represent the so called lone pairs. Electron in the first energy shellelectron in the third energy shell.
The electron in the third energy shell has more potential energy because of its relative distance from the nucleus. For example the bond between the oxygen and hydrogen atoms of a water molecule is a polar covalent bond. Make an electron distribution diagram of water.
The chemical properties of the elements reflect their electron configurations. Sketch a water molecule showing oxygens electron shells and the covalently shared electrons. Water is considered a polar molecule because oxygen is more electronegative than hydrogen and therefore the shared electrons are pulled more toward oxygen.
Make an electron distribution diagram of water. Yes this can happen under the right conditions. It is a scientifically sound well laid out collection of articles on water and its structure which should answer any of your questions.
Request pdf on researchgate electron distribution in water the x ray structure factor of water measured under ambient conditions with synchrotron radiation is compared with those predicted on. Label the regions that are more positive or more negative. Water structure and properties is a web site developed by martin chaplin at south bank university in england.
Glucose has more potential energy because of its structure. Which element is most electronegative. Does hot water freeze faster than cold water.
This is a very important concept. Why is water considered a polar molecule. Then draw a second water molecule and indicate a hydrogen bond between the two.
 Lewis Dot Diagram Worksheet Answers Electron Distribution Diagram
Lewis Dot Diagram Worksheet Answers Electron Distribution Diagram
 Electron Distribution Diagram O2 Wiring Diagram And Electrical
Electron Distribution Diagram O2 Wiring Diagram And Electrical
 Electron Distribution Diagram For Copper Www Culturebee Co
Electron Distribution Diagram For Copper Www Culturebee Co
 Review Of Chemistry And The Properties Of Water Quick Comments On
Review Of Chemistry And The Properties Of Water Quick Comments On
 Spontaneous Droplets Gyrating Via Asymmetric Self Splitting On
Spontaneous Droplets Gyrating Via Asymmetric Self Splitting On
Electron Distribution Diagram Of Carbon Wiring Diagrams
 Electron Distribution Diagram Water
Electron Distribution Diagram Water

 Ground State Electron Configuration Definition Example Video
Ground State Electron Configuration Definition Example Video
Electron Distribution Diagram Of Carbon Applied Sciences Free Full
 Draw The Electron Distribution Diagram Of A Water Molecule
Draw The Electron Distribution Diagram Of A Water Molecule
 Ground State Electron Configuration Definition Example Video
Ground State Electron Configuration Definition Example Video
Iron Has 26 Electrons But Why Is Its Valency 2 Quora
 Chemistry I Atoms And Molecules
Chemistry I Atoms And Molecules
 Why Is Water A Good Solvent Socratic
Why Is Water A Good Solvent Socratic
Elegant Electron Distribution Diagram Water Lewis Dot Diagram For
50 Beautiful Oxygen Electron Shell Diagram Abdpvtltd Com
Introduction To Molecular Orbital Theory
Lewis Dot Diagram For Water Unique Electron Distribution Diagram

0 Response to "Electron Distribution Diagram Of Water"
Post a Comment